Physiological modulation of rabphilin phosphorylation
- PMID: 11466418
- PMCID: PMC6762674
- DOI: 10.1523/JNEUROSCI.21-15-05473.2001
Physiological modulation of rabphilin phosphorylation
Abstract
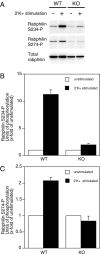
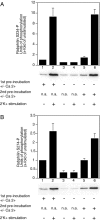
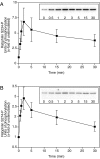
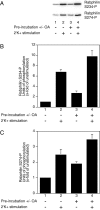
The dynamic modulation of protein function by phosphorylation plays an important role in regulating synaptic plasticity. Several proteins involved in synaptic transmission have been shown to be targets of protein kinases and phosphatases. A thorough analysis of the physiological role of these modifications has been hampered by the lack of reagents that specifically recognize the phosphorylated states of these proteins. In this study we analyze the physiological modulation of rabphilin using phosphospecific antibodies. We show that phosphorylation on serine-234 and serine-274 of rabphilin is dynamically regulated both under basal and stimulated conditions by the activity of kinases and phosphatases. The two sites are differentially phosphorylated by the stimulation of various kinases, suggesting a possible convergence of different pathways to modulate the function of the protein. Maximal stimulation was observed under plasma membrane-depolarizing conditions that trigger synaptic vesicle exocytosis. The increase in phosphorylation was critically dependent on external Ca(2+) and on the presence of Rab3a, a small GTPase that recruits rabphilin to synaptic vesicles. The rapid phosphorylation and dephosphorylation during and after stimulation demonstrates the transient nature of the modification. Our results indicate that rabphilin is phosphorylated on synaptic vesicles by Ca(2+)-dependent kinases that become active in synaptic terminals during exocytosis. We have found that phosphorabphilin has a reduced affinity for membranes; we therefore propose that the modulation of the membrane association of rabphilin has a role in the synaptic vesicle life cycle, perhaps in vesicle mobilization in preparation for subsequent rounds of neurotransmission.
Figures



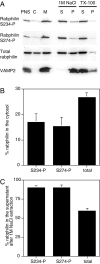
References
-
- Alger BE, Dhanjal SS, Dingledine R, Garthwaite J, Henderson G, King GL, Lipton P, North A, Schwartzkroin PA, Sears TA, Segal M, Whittingham TS, Williams J. In: Brain slices (Dingledine R, ed), pp 381–437. New York; Plenum: 1984.
-
- Arribas M, Regazzi R, Garcia E, Wollheim CB, De Camilli P. The stimulatory effect of rabphilin 3a on regulated exocytosis from insulin-secreting cells does not require an association-dissociation cycle with membranes mediated by Rab 3. Eur J Cell Biol. 1997;74:209–216. - PubMed
-
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. - PubMed
-
- Chung SH, Takai Y, Holz RW. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. Studies in adrenal chromaffin cells. J Biol Chem. 1995;270:16714–16718. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
