Role of IL-1alpha and IL-1beta in ischemic brain damage
- PMID: 11466424
- PMCID: PMC6762680
- DOI: 10.1523/JNEUROSCI.21-15-05528.2001
Role of IL-1alpha and IL-1beta in ischemic brain damage
Erratum in
- J Neurosci 2001 Sep 1;21(17):1a
Abstract
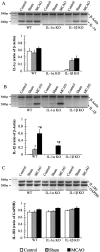
The cytokine interleukin-1 (IL-1) has been strongly implicated in the pathogenesis of ischemic brain damage. Evidence to date suggests that the major form of IL-1 contributing to ischemic injury is IL-1beta rather than IL-1alpha, but this has not been tested directly. The objective of the present study was to compare the effects of transient cerebral ischemia [30 min middle cerebral artery occlusion (MCAO)] on neuronal injury in wild-type (WT) mice and in IL-1alpha, IL-1beta, or both IL-1alpha and IL-1beta knock-out (KO) mice. Mice lacking both forms of IL-1 exhibited dramatically reduced ischemic infarct volumes compared with wild type (total volume, 70%; cortex, 87% reduction). Ischemic damage compared with WT mice was not significantly altered in mice lacking either IL-1alpha or IL-1beta alone. IL-1beta mRNA, but not IL-1alpha or the IL-1 type 1 receptor, was strongly induced by MCAO in WT and IL-1alpha KO mice. Administration (intracerebroventricularly) of recombinant IL-1 receptor antagonist significantly reduced infarct volume in WT (-32%) and IL-1alpha KO (-48%) mice, but had no effect on injury in IL-1beta or IL-1alpha/beta KO mice. These data confirm that IL-1 plays a major role in ischemic brain injury. They also show that chronic deletion of IL-1alpha or IL-1beta fails to influence brain damage, probably because of compensatory changes in the IL-1 system in IL-1alpha KO mice and changes in IL-1-independent mediators of neuronal death in IL-1beta KO mice.
Figures
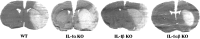
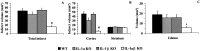
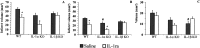
References
-
- Akita K, Ohtsuki T, Nukada Y, Tanimoto T, Namba M, Okura T, Takakura-Yamamoto R, Torigoe K, Gu Y, Su MS, Fujii M, Satoh-Itoh M, Yamamoto K, Kohno K, Ikeda M, Kurimoto M. Involvement of caspase-1 and caspase-3 in the production and processing of mature human interleukin 18 in monocytic THP.1 cells. J Biol Chem. 1997;272:26595–26603. - PubMed
-
- Alheim K, Chai Z, Fantuzzi G, Hasanvan H, Malinowsky D, Di Santo E, Ghezzi P, Dinarello CA, Bartfai T. Hyperresponsive febrile reactions to interleukin (IL)-1alpha and IL-1beta, and altered brain cytokine mRNA and serum cytokine levels, in IL-1beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:2681–2686. - PMC - PubMed
-
- Allan SM, Lawrence CB, Grundy RP, Stroemer RP, Rothwell NJ. Sites and mechanisms of interleukin-1 action. In: Krieglstein J, Oberpichler-Schwenk H, editors. Pharmacology of cerebral ischemia. Medpharm Scientific; Stuttgart: 1998. pp. 395–399.
-
- Betz AL, Yang GY, Davidson BL. Attenuation of stroke size in rats using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist in brain. J Cereb Blood Flow Metab. 1995;15:547–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
