Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane
- PMID: 11466426
- PMCID: PMC6762635
- DOI: 10.1523/JNEUROSCI.21-15-05546.2001
Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane
Abstract
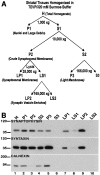
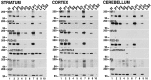
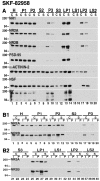
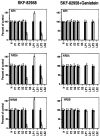
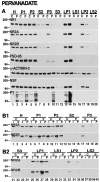
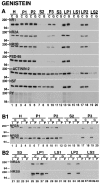
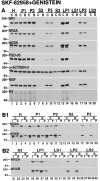
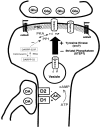
Recent work has shown substantial alterations in NMDA receptor subunit expression, assembly, and phosphorylation in the dopamine-depleted striatum of a rodent 6-hydroxydopamine model of Parkinson's disease. These modifications are hypothesized to result from the trafficking of NMDA receptors between subcellular compartments. Here we show that in rat striatal tissues the NR2A and NR2B subunits in the synaptosomal membrane, and not those in the light membrane and synaptic vesicle-enriched compartments, are tyrosine phosphorylated. The dopamine D1 receptor agonist SKF-82958 produces (1) an increase in NR1, NR2A, and NR2B proteins in the synaptosomal membrane fraction; (2) a decrease in NR1, NR2A, and NR2B proteins in the light membrane and synaptic vesicle-enriched fractions; and (3) an increase in the tyrosine phosphorylation of NR2A and NR2B in the synaptosomal membrane compartment. The protein phosphatase inhibitor pervanadate reproduces the alterations in subcellular distribution and phosphorylation, whereas the effects of the dopamine D1 receptor agonist are blocked by genistein, a protein tyrosine kinase inhibitor. Dopamine D1 receptor agonist treatment does not change the subcellular distribution of the AMPA receptor subunits GluR1 or GluR2/3 in the striatum and has no effect on cortical or cerebellar NMDA receptor subunits. These data reveal a rapid dopamine D1 receptor- and tyrosine kinase-dependent trafficking of striatal NMDA receptors between intracellular and postsynaptic sites. The subcellular trafficking of striatal NMDA receptors may play a significant role both in the pathogenesis of Parkinson's disease and in the development of adverse effects of chronic dopaminergic therapy in parkinsonian patients.
Figures



References
-
- Barinaga M. Secrets of secretion revealed. Science. 1993;260:487–489. - PubMed
-
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. - PubMed
-
- Blanchet PJ, Papa SM, Metman LV, Mouradian MM, Chase TN. Modulation of levodopa-induced motor response complications by NMDA antagonists in Parkinson's disease. Neurosci Biobehav Rev. 1997;21:447–453. - PubMed
-
- Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
