Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits
- PMID: 11466442
- PMCID: PMC6762634
- DOI: 10.1523/JNEUROSCI.21-15-05703.2001
Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits
Abstract
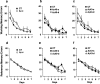
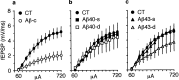
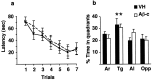
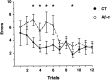
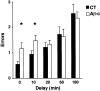
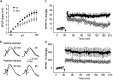
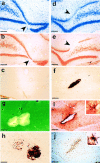
We injected a combination of the beta-amyloids (Abetas) Abeta40 and Abeta43 to "seed" formation of amyloid deposits in the dorsal dentate gyrus of rats in vivo, on the basis of a theory of Jarrett and Landsbury (1993). Rats were tested on several different learning tasks, and synaptic transmission and plasticity were assessed in vivo. Between 7 and 16 weeks after injection, we found aggregated amyloid material, reactive astrocytosis, microgliosis, and cell loss around the sites of injection. Rats were impaired specifically in working memory type tasks in accordance with the type of memory deficit observed in the early stages of Alzheimer's disease. Synaptic transmission and long-term potentiation, a candidate cellular mechanism for memory, were severely impaired in vivo. Injections of the same dose of fragments individually did not induce these effects. These findings suggest that aggregated amyloid material induces cognitive deficits similar to those observed in the early phases of Alzheimer's disease via an alteration in neuronal transmission and plasticity.
Figures



References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, George-Hyslop PS, Cordell B, Fraser P, De Strooper B. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-Golgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. - PMC - PubMed
-
- Ball MJ, Fisman M, Hachinski V, Blume W, Fox A, Kral VA, Kirshen AJ, Fox H, Mersky H. A new definition of Alzheimer's disease: a hippocampal dementia. Lancet. 1985;1:14–16. - PubMed
-
- Barone S, Tandon P, McGinty JF, Tilson HA. The effects of NGF and fetal cell transplants on spatial learning after intradentate administration of colchicine. Exp Neurol. 1991;114:351–363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
