Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI
- PMID: 11466443
- PMCID: PMC6762662
- DOI: 10.1523/JNEUROSCI.21-15-05715.2001
Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI
Abstract
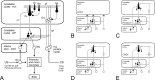
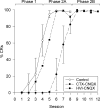
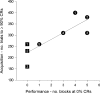
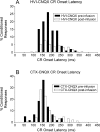
Classical conditioning of the nictitating membrane response (NMR)/eyeblink response of rabbits is a simple form of cerebellar-dependent, associative motor learning. Reversible inactivations of the cerebellar nuclei and inferior olive have implicated the olivo-cortico-nuclear loop in the acquisition of nictitating membrane conditioning, but the role of the cerebellar cortex in acquisition has not been tested directly. Here we have used local infusions of the water-soluble, disodium salt of 6-cyano-7-nitroquinoxaline-2,3-dione reversibly to block cerebellar cortical AMPA/kainate receptors in lobule HVI during acquisition training. After the drug effects dissipated, there was no evidence that acquisition had taken place; the subjects behaved as if naive. Further training without inactivation then allowed normal acquisition, and further inactivations during performance of conditioned responses abolished these established responses. There was a strong correlation between the inactivation effects on acquisition and subsequent inactivation effects on performance, indicating that the same eyeblink-control cortical microzones are engaged in learning and expressing this behavior. The cortical component of the olivo-cortico-nuclear loop is essential for acquisition of classically conditioned nictitating membrane response learning, and eyeblink control areas in HVI are critical. Our findings are consistent with models of cerebellar learning that assign essential plasticity to the cortex or to a distribution between levels in olivo-cortico-nuclear modules.
Figures


References
-
- Albus J. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Andersson G, Hesslow G. Inferior olive excitability after high frequency climbing fibre activation in the cat. Exp Brain Res. 1987;67:523–532. - PubMed
-
- Andersson G, Garwicz M, Hesslow G. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Exp Brain Res. 1988;72:450–456. - PubMed
-
- Colin P, Manil J, Desclin JC. The olivocerebellar system. 1. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187:3–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
