Reversible formation of on-pathway macroscopic aggregates during the folding of maltose binding protein
- PMID: 11468360
- PMCID: PMC2374092
- DOI: 10.1110/ps.8101
Reversible formation of on-pathway macroscopic aggregates during the folding of maltose binding protein
Abstract
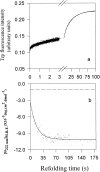
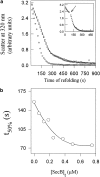
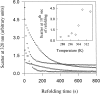
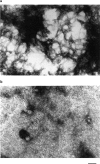
Maltose binding protein (MBP) is widely used as a model for protein folding and export studies. We show here that macroscopic aggregates form transiently during the refolding of MBP at micromolar protein concentrations. Disaggregation occurs spontaneously without any aid, and the refolded material has structure and activity identical to those of the native, nondenatured protein. A considerable fraction of protein undergoing folding partitions into the aggregate phase and can be manually separated from the soluble phase by centrifugation. The separated MBP precipitate can be resolubilized and yields active, refolded protein. This demonstrates that both the soluble and aggregate phases contribute to the final yield of refolded protein. SecB, the cognate Escherichia coli cytosolic chaperone in vivo for MBP, reduces but does not entirely prevent aggregation, whereas GroEL and a variety of other control proteins have no effect. Kinetic studies using a variety of spectroscopic probes show that aggregation occurs through a collapsed intermediate with some secondary structure. The aggregate formed during refolding can convert directly to a near native state without going through the unfolded state. Further, optical and electron microscopic studies indicate that the MBP precipitate is not an amyloid.
Figures

References
-
- Agashe, V.R., Shastry, M.C., and Udgaonkar, J.B. 1995. Initial hydrophobic collapse in the folding of barstar. Nature 377 754–757. - PubMed
-
- Anfinsen, C.B. 1973. Principles that govern the folding of protein chains. Science 181 223–230. - PubMed
-
- Betton, J. and Hofnung, M. 1996. Folding of a mutant maltose-binding protein of Escherichia coli which forms inclusion bodies. J. Biol. Chem. 271 8046–8052. - PubMed
-
- Betton, J.M., Sassoon, N., Hofnung, M., and Laurent, M. 1998. Degradation versus aggregation of misfolded maltose-binding protein in the periplasm of Escherichia coli. J. Biol. Chem. 273 8897–8902. - PubMed
-
- Brems, D.N. 1990. Deciphering the second half of the genetic code. In Protein folding (eds. L.M. Gierasch and J. King), pp. 129–135. American Association For The Advancement of Science.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

