Hsp90: a specialized but essential protein-folding tool
- PMID: 11470816
- PMCID: PMC2150759
- DOI: 10.1083/jcb.200104079
Hsp90: a specialized but essential protein-folding tool
Abstract
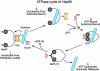
Hsp90 is unique among molecular chaperones. The majority of its known substrates are signal transduction proteins, and recent work indicates that it uses a novel protein-folding strategy.
Figures


Similar articles
-
Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system.EMBO J. 2003 Jul 15;22(14):3613-23. doi: 10.1093/emboj/cdg362. EMBO J. 2003. PMID: 12853476 Free PMC article.
-
Hsp90: chaperoning signal transduction.J Cell Physiol. 2001 Sep;188(3):281-90. doi: 10.1002/jcp.1131. J Cell Physiol. 2001. PMID: 11473354 Review.
-
Evolution and function of diverse Hsp90 homologs and cochaperone proteins.Biochim Biophys Acta. 2012 Mar;1823(3):607-13. doi: 10.1016/j.bbamcr.2011.09.020. Epub 2011 Oct 8. Biochim Biophys Acta. 2012. PMID: 22008467 Review.
-
ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo.EMBO J. 1998 Aug 17;17(16):4829-36. doi: 10.1093/emboj/17.16.4829. EMBO J. 1998. PMID: 9707442 Free PMC article.
-
The HSP90 chaperone machinery.Nat Rev Mol Cell Biol. 2017 Jun;18(6):345-360. doi: 10.1038/nrm.2017.20. Epub 2017 Apr 21. Nat Rev Mol Cell Biol. 2017. PMID: 28429788 Review.
Cited by
-
Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson's Disease.Int J Mol Sci. 2023 Nov 12;24(22):16233. doi: 10.3390/ijms242216233. Int J Mol Sci. 2023. PMID: 38003423 Free PMC article. Review.
-
The Hsp90 Co-chaperones Sti1, Aha1, and P23 Regulate Adaptive Responses to Antifungal Azoles.Front Microbiol. 2016 Oct 5;7:1571. doi: 10.3389/fmicb.2016.01571. eCollection 2016. Front Microbiol. 2016. PMID: 27761133 Free PMC article.
-
Genome-scale co-evolutionary inference identifies functions and clients of bacterial Hsp90.PLoS Genet. 2013;9(7):e1003631. doi: 10.1371/journal.pgen.1003631. Epub 2013 Jul 11. PLoS Genet. 2013. PMID: 23874229 Free PMC article.
-
From Small Peptides to Large Proteins against Alzheimer'sDisease.Biomolecules. 2022 Sep 22;12(10):1344. doi: 10.3390/biom12101344. Biomolecules. 2022. PMID: 36291553 Free PMC article. Review.
-
Degradable Cross-Linked Nanoassemblies as Drug Carriers for Heat Shock Protein 90 Inhibitor 17-N-Allylamino-17-demethoxy-geldanamycin.Pharmaceuticals (Basel). 2011 Sep 26;4(10):1281-1292. doi: 10.3390/ph4101281. Pharmaceuticals (Basel). 2011. PMID: 27721325 Free PMC article.
References
-
- Arbeitman, M.N., and D.S. Hogness. 2000. Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell. 101:67–77. - PubMed
-
- Argon, Y., and B.B. Simen. 1999. Grp94, an ER chaperone with protein and peptide binding properties. Semin. Cell. Dev. Biol. 10:495–505. - PubMed
-
- Ban, C., M. Junop, and W. Yang. 1999. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 97:85–97. - PubMed
-
- Berger, J.M., S.J. Gamblin, S.C. Harrison, and J.C. Wang. 1996. Structure and mechanism of DNA topoisomerase II. Nature. 379:225–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

