Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney
- PMID: 11470831
- PMCID: PMC2150762
- DOI: 10.1083/jcb.200103069
Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney
Abstract
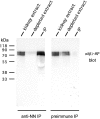
The epithelial-mesenchymal interactions required for kidney organogenesis are disrupted in mice lacking the integrin alpha8beta1. None of this integrin's known ligands, however, appears to account for this phenotype. To identify a more relevant ligand, a soluble integrin alpha8beta1 heterodimer fused to alkaline phosphatase (AP) has been used to probe blots and cDNA libraries. In newborn mouse kidney extracts, alpha8beta1-AP detects a novel ligand of 70-90 kD. This protein, named nephronectin, is an extracellular matrix protein with five EGF-like repeats, a mucin region containing a RGD sequence, and a COOH-terminal MAM domain. Integrin alpha8beta1 and several additional RGD-binding integrins bind nephronectin. Nephronectin mRNA is expressed in the ureteric bud epithelium, whereas alpha8beta1 is expressed in the metanephric mesenchyme. Nephronectin is localized in the extracellular matrix in the same distribution as the ligand detected by alpha8beta1-AP and forms a complex with alpha8beta1 in vivo. Thus, these results strongly suggest that nephronectin is a relevant ligand mediating alpha8beta1 function in the kidney. Nephronectin is expressed at numerous sites outside the kidney, so it may also have wider roles in development. The approaches used here should be generally useful for characterizing the interactions of novel extracellular matrix proteins identified through genomic sequencing projects.
Figures





Comment in
-
Mystery solved: discovery of a novel integrin ligand in the developing kidney.J Cell Biol. 2001 Jul 23;154(2):257-9. doi: 10.1083/jcb.200106124. J Cell Biol. 2001. PMID: 11470814 Free PMC article. Review.
References
-
- Buchner G, U. Orfanelli, N. Quaderi, M.T. Bassi, G. Andolfi, A. Ballabio, and B. Franco. 2000. Identification of a new EGF-repeat-containing gene from human Xp22: a candidate for developmental disorders. Genomics. 65:16–23. - PubMed
-
- Denda, S., U. Müller, K.L. Crossin, H.P. Erickson, and L.F. Reichardt. 1998. a. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin α8β1 receptor interactions with tenascin: murine α8β1 binds to the RGD site in tenascin-c fragments, but not to native tenascin-c. Biochemistry. 37:5464–5474. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

