Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor
- PMID: 11470832
- PMCID: PMC2150768
- DOI: 10.1083/jcb.200103103
Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor
Abstract
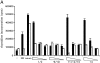
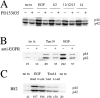
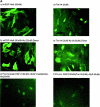
Signaling through growth factor receptors controls such diverse cell functions as proliferation, migration, and differentiation. A critical question has been how the activation of these receptors is regulated. Most, if not all, of the known ligands for these receptors are soluble factors. However, as matrix components are highly tissue-specific and change during development and pathology, it has been suggested that select growth factor receptors might be stimulated by binding to matrix components. Herein, we describe a new class of ligand for the epidermal growth factor (EGF) receptor (EGFR) found within the EGF-like repeats of tenascin-C, an antiadhesive matrix component present during organogenesis, development, and wound repair. Select EGF-like repeats of tenascin-C elicited mitogenesis and EGFR autophosphorylation in an EGFR-dependent manner. Micromolar concentrations of EGF-like repeats induced EGFR autophosphorylation and activated extracellular signal-regulated, mitogen-activated protein kinase to levels comparable to those induced by subsaturating levels of known EGFR ligands. EGFR-dependent adhesion was noted when the ligands were tethered to inert beads, simulating the physiologically relevant presentation of tenascin-C as hexabrachion, and suggesting an increase in avidity similar to that seen for integrin ligands upon surface binding. Specific binding to EGFR was further established by immunofluorescence detection of EGF-like repeats bound to cells and cross-linking of EGFR with the repeats. Both of these interactions were abolished upon competition by EGF and enhanced by dimerization of the EGF-like repeat. Such low affinity behavior would be expected for a matrix-"tethered" ligand; i.e., a ligand which acts from the matrix, presented continuously to cell surface EGF receptors, because it can neither diffuse away nor be internalized and degraded. These data identify a new class of "insoluble" growth factor ligands and a novel mode of activation for growth factor receptors.
Figures







References
-
- Akiyama, S.K., and K.M. Yamada. 1985. Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional biological assays for the purified cell-binding domain of fibronectin. J. Biol. Chem. 260:4492–4500. - PubMed
-
- Anklesaria, P., J. Teixido, M. Laiho, J.H. Pierce, J.S. Greenberger, and J. Massague. 1990. Cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc. Natl. Acad. Sci. USA. 87:3289–3293. - PMC - PubMed
-
- Banerjee, P., D.J. Irvine, A.M. Mayes, and L.G. Griffith. 2000. Polymer latexes for controlling cell adhesion and receptor-mediated interactions. J. Biomed. Mater. Res. 50:331–339. - PubMed
-
- Bhalla, U.S., and R. Iyengar. 1999. Emergent properties of networks of biological signaling pathways. Science. 283:381–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

