DNA looping in the RNA polymerase I enhancesome is the result of non-cooperative in-phase bending by two UBF molecules
- PMID: 11470882
- PMCID: PMC55825
- DOI: 10.1093/nar/29.15.3241
DNA looping in the RNA polymerase I enhancesome is the result of non-cooperative in-phase bending by two UBF molecules
Abstract
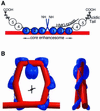
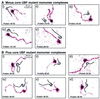
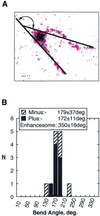
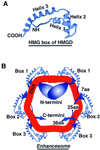
The so-called upstream binding factor (UBF) is required for the initial step in formation of an RNA polymerase I initiation complex. This function of UBF correlates with its ability to induce the ribosomal enhancesome, a structure which resembles in its mass and DNA content the nucleosome of chromatin. DNA looping in the enhancesome is probably the result of six in-phase bends induced by the HMG boxes of a UBF dimer. Here we show that insertion/deletion mutations in the basic peptide linker lying between the N-terminal dimerisation domain and the first HMG box of Xenopus UBF prevent the DNA looping characteristic of the enhancesome. Using these mutants we demonstrate that (i) the enhancesome structure does not depend on tethering of the entering and exiting DNA duplexes, (ii) UBF monomers induce hemi-enhancesomes, bending the DNA by 175 +/- 24 degrees and (iii) two hemi-enhancesomes are precisely phased by UBF dimerisation. We use this and previous data to refine the existing enhancesome model and show that HMG boxes 1 and 2 of UBF lie head-to-head along the DNA.
Figures


References
-
- Bazett-Jones D.P., Leblanc,B., Herfort,M. and Moss,T. (1994) Short-range DNA looping by the Xenopus HMG-box transcription factor, xUBF. Science, 264, 1134–1137. - PubMed
-
- Moss T. and Stefanovsky,V.Y. (1995) Promotion and regulation of ribosomal transcription in eukaryotes by RNA Polymerase I. In Cohn,W.E. and Moldave,K. (eds), Progress in Nucleic Acids and Molecular Biology. Academic Press, San Diego, CA, pp. 25–66. - PubMed
-
- Moss T., Stefanovsky,V.Y. and Pelletier,G. (1998) The structural and architectural role of Upstream Binding Factor, UBF. In Paule,M.R. (ed.), Transcription of Ribosomal Genes by Eukaryotic RNA Polymerase I. Landes Bioscience, Austin, TX, pp. 75–94.

