A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver
- PMID: 11470916
- PMCID: PMC55382
- DOI: 10.1073/pnas.161284298
A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver
Abstract
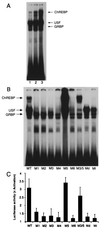
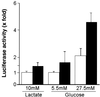
Carbohydrates mediate their conversion to triglycerides in the liver by promoting both rapid posttranslational activation of rate-limiting glycolytic and lipogenic enzymes and transcriptional induction of the genes encoding many of these same enzymes. The mechanism by which elevated carbohydrate levels affect transcription of these genes remains unknown. Here we report the purification and identification of a transcription factor that recognizes the carbohydrate response element (ChRE) within the promoter of the L-type pyruvate kinase (LPK) gene. The DNA-binding activity of this ChRE-binding protein (ChREBP) in rat livers is specifically induced by a high carbohydrate diet. ChREBP's DNA-binding specificity in vitro precisely correlates with promoter activity in vivo. Furthermore, forced ChREBP overexpression in primary hepatocytes activates transcription from the L-type Pyruvate kinase promoter in response to high glucose levels. The DNA-binding activity of ChREBP can be modulated in vitro by means of changes in its phosphorylation state, suggesting a possible mode of glucose-responsive regulation. ChREBP is likely critical for the optimal long-term storage of excess carbohydrates as fats, and may contribute to the imbalance between nutrient utilization and storage characteristic of obesity.
Figures


References
-
- Neel J N. Nutr Rev. 1999;57:S2–S9. - PubMed
-
- Troiano R P, Flegal K M. Int J Obes Relat Metab Disord. 1999;23:S22–S27. - PubMed
-
- Goodridge A G. Annu Rev Nutr. 1987;7:157–185. - PubMed
-
- Granner D, Pilkis S. J Biol Chem. 1990;265:10173–10176. - PubMed
-
- Girard J, Ferré P, Foufelle F. Annu Rev Nutr. 1997;17:325–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

