Presence of mitochondria-type ribosomes outside mitochondria in germ plasm of Drosophila embryos
- PMID: 11470924
- PMCID: PMC55385
- DOI: 10.1073/pnas.171286998
Presence of mitochondria-type ribosomes outside mitochondria in germ plasm of Drosophila embryos
Abstract
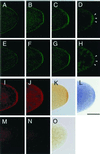
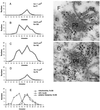
Mitochondrially encoded large and small ribosomal RNAs (mtlrRNA and mtsrRNA) are transported out of mitochondria to polar granules, the distinctive organelles of germ plasm in Drosophila. Reduction of the extramitochondrial mtlrRNA amount leads to the failure of embryos to form the germ-line progenitors, or pole cells, suggesting that mtlrRNA, along with mtsrRNA, functions on the polar granules to specify the germ line. In this study, we provide several lines of evidence showing that there are mitochondria-type ribosomes on the polar granules during a short period before pole cell formation. Our ultrastructural analysis reveals that these ribosomes include both mitochondrial rRNAs and at least two mitochondrial ribosomal proteins (S12 and L7/L12). Furthermore, these ribosomes are integrated into well developed polysomes on the surface of polar granules. We propose that translation dependent on mitochondria-type ribosomes is an important mechanism underlying germ-line formation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

