Biotransformation of various substituted aromatic compounds to chiral dihydrodihydroxy derivatives
- PMID: 11472901
- PMCID: PMC93025
- DOI: 10.1128/AEM.67.8.3333-3339.2001
Biotransformation of various substituted aromatic compounds to chiral dihydrodihydroxy derivatives
Abstract
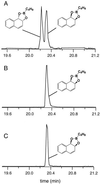
The biotransformation of four different classes of aromatic compounds by the Escherichia coli strain DH5alpha(pTCB 144), which contained the chlorobenzene dioxygenase (CDO) from Pseudomonas sp. strain P51, was examined. CDO oxidized biphenyl as well as monochlorobiphenyls to the corresponding cis-2,3-dihydro-2,3-dihydroxy derivatives, whereby oxidation occurred on the unsubstituted ring. No higher substituted biphenyls were oxidized. The absolute configurations of several monosubstituted cis-benzene dihydrodiols formed by CDO were determined. All had an S configuration at the carbon atom in meta position to the substituent on the benzene nucleus. With one exception, the enantiomeric excess of several 1,4-disubstituted cis-benzene dihydrodiols formed by CDO was higher than that of the products formed by two toluene dioxygenases. Naphthalene was oxidized to enantiomerically pure (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene. All absolute configurations were identical to those of the products formed by toluene dioxygenases of Pseudomonas putida UV4 and P. putida F39/D. The formation rate of (+)-cis-(1R,2S)-dihydroxy-1,2-dihydronaphthalene was significantly higher (about 45 to 200%) than those of several monosubstituted cis-benzene dihydrodiols and more than four times higher than the formation rate of cis-benzene dihydrodiol. A new gas chromatographic method was developed to determine the enantiomeric excess of the oxidation products.
Figures
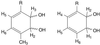

References
-
- Allen C C R, Boyd D R, Dalton H, Sharma N D, Brannigan I, Kerley N A, Sheldrake G N, Taylor S C. Enantioselective bacterial biotransformation routes to cis-diol metabolites of monosubstituted benzenes, naphthalene and benzocycloalkanes of either absolute configuration. J Chem Soc Chem Commun. 1995;1995:117–118.
-
- Bestetti G, Bianchi D, Bosetti A, Di Gennaro P, Galli E, Leoni B, Pelizzoni F, Sello G. Bioconversion of substituted naphthalenes to the corresponding 1,2-dihydro-1,2-dihydroxy derivatives. Determination of the regio-and stereochemistry of the oxidation reactions. Appl Microbiol Biotechnol. 1995;44:306–313.
-
- Boyd D R, Dorrity M R J, Hand M V, Malone J F, Sharma N D, Dalton H, Gray D J, Sheldrake G N. Enantiomeric excess and absolute configuration determination of cis-dihydrodiols from bacterial metabolism of monocyclic arenes. J Am Chem Soc. 1991;113:666–667.
-
- Boyd D R, Sharma N D, Hand M V, Groocock M R, Kerley N A, Dalton H, Chima J, Sheldrake G N. Stereodirecting substituent effects during enzyme-catalysed synthesis of cis-dihydrodiol metabolites of 1,4-disubstituted benzene substrates. J Chem Soc Chem Commun. 1993;32:974–976.
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

