Altered protein expression of Streptococcus oralis cultured at low pH revealed by two-dimensional gel electrophoresis
- PMID: 11472910
- PMCID: PMC93034
- DOI: 10.1128/AEM.67.8.3396-3405.2001
Altered protein expression of Streptococcus oralis cultured at low pH revealed by two-dimensional gel electrophoresis
Abstract
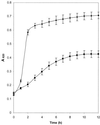
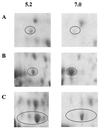
Streptococcus oralis is the predominant aciduric nonmutans streptococcus isolated from the human dentition, but the role of this organism in the initiation and progression of dental caries has yet to be established. To identify proteins that are differentially expressed by S. oralis growing under conditions of low pH, soluble cellular proteins extracted from bacteria grown in batch culture at pH 5.2 or 7.0 were analyzed by two-dimensional (2-D) gel electrophoresis. Thirty-nine proteins had altered expression at low pH; these were excised, digested with trypsin using an in-gel protocol, and further analyzed by peptide mass fingerprinting using matrix-assisted laser desorption ionization mass spectrometry. The resulting fingerprints were compared with the genomic database for Streptococcus pneumoniae, an organism that is phylogenetically closely related to S. oralis, and putative functions for the majority of these proteins were determined on the basis of functional homology. Twenty-eight proteins were up-regulated following growth at pH 5.2; these included enzymes of the glycolytic pathway (glyceraldehyde-3-phosphate dehydrogenase and lactate dehydrogenase), the polypeptide chains comprising ATP synthase, and proteins that are considered to play a role in the general stress response of bacteria, including the 60-kDa chaperone, Hsp33, and superoxide dismutase, and three distinct ABC transporters. These data identify, for the first time, gene products that may be important in the survival and proliferation of nonmutans aciduric S. oralis under conditions of low pH that are likely to be encountered by this organism in vivo.
Figures


Similar articles
-
Effect of acidic pH on expression of surface-associated proteins of Streptococcus oralis.Appl Environ Microbiol. 2003 Sep;69(9):5290-6. doi: 10.1128/AEM.69.9.5290-5296.2003. Appl Environ Microbiol. 2003. PMID: 12957916 Free PMC article.
-
Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions.Appl Environ Microbiol. 2002 May;68(5):2382-90. doi: 10.1128/AEM.68.5.2382-2390.2002. Appl Environ Microbiol. 2002. PMID: 11976112 Free PMC article.
-
Identification and characterization of Porphyromonas gingivalis client proteins that bind to Streptococcus oralis glyceraldehyde-3-phosphate dehydrogenase.Infect Immun. 2013 Mar;81(3):753-63. doi: 10.1128/IAI.00875-12. Epub 2012 Dec 21. Infect Immun. 2013. PMID: 23264054 Free PMC article.
-
Sequential deglycosylation and utilization of the N-linked, complex-type glycans of human alpha1-acid glycoprotein mediates growth of Streptococcus oralis.Glycobiology. 1999 May;9(5):469-79. doi: 10.1093/glycob/9.5.469. Glycobiology. 1999. PMID: 10207179
-
Differential proteomic analysis in the study of prokaryotes stress resistance.Ann Ist Super Sanita. 2005;41(4):459-68. Ann Ist Super Sanita. 2005. PMID: 16569914 Review.
Cited by
-
Timing and specificity of cotranslational nascent protein modification in bacteria.Proc Natl Acad Sci U S A. 2019 Nov 12;116(46):23050-23060. doi: 10.1073/pnas.1912264116. Epub 2019 Oct 30. Proc Natl Acad Sci U S A. 2019. PMID: 31666319 Free PMC article.
-
Extracellular arginine aminopeptidase from Streptococcus gordonii FSS2.Infect Immun. 2002 Feb;70(2):836-43. doi: 10.1128/IAI.70.2.836-843.2002. Infect Immun. 2002. PMID: 11796618 Free PMC article.
-
Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809.J Bacteriol. 2005 Aug;187(16):5799-808. doi: 10.1128/JB.187.16.5799-5808.2005. J Bacteriol. 2005. PMID: 16077128 Free PMC article.
-
Effects of sucrose on the extracellular matrix of plaque-like biofilm formed in vivo, studied by proteomic analysis.Caries Res. 2008;42(6):435-43. doi: 10.1159/000159607. Epub 2008 Oct 3. Caries Res. 2008. PMID: 18832830 Free PMC article. Clinical Trial.
-
A Comparative Study of the Outer Membrane Proteome from an Atypical and a Typical Enteropathogenic Escherichia coli.Open Microbiol J. 2011;5:83-90. doi: 10.2174/1874285801105010083. Epub 2011 Jul 20. Open Microbiol J. 2011. PMID: 21804903 Free PMC article.
References
-
- Bentley R W, Leigh J A, Collins M D. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int J Syst Bacteriol. 1991;41:487–494. - PubMed
-
- Bernhardt J, Volker U, Volker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology. 1997;143:999–1017. - PubMed
-
- Brailsford S R, Byrne R W, Adams S, Zoitoppoulos L, Allison C, Beighton D. Investigation of the aciduric microflora of plaque. Caries Res. 1999;33:290.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

