Malaria rapid diagnostic devices: performance characteristics of the ParaSight F device determined in a multisite field study
- PMID: 11474008
- PMCID: PMC88255
- DOI: 10.1128/JCM.39.8.2884-2890.2001
Malaria rapid diagnostic devices: performance characteristics of the ParaSight F device determined in a multisite field study
Abstract
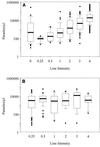
Microscopic detection of parasites has been the reference standard for malaria diagnosis for decades. However, difficulty in maintaining required technical skills and infrastructure has spurred the development of several nonmicroscopic malaria rapid diagnostic devices based on the detection of malaria parasite antigen in whole blood. The ParaSight F test is one such device. It detects the presence of Plasmodium falciparum-specific histidine-rich protein 2 by using an antigen-capture immunochromatographic strip format. The present study was conducted at outpatient malaria clinics in Iquitos, Peru, and Maesod, Thailand. Duplicate, blinded, expert microscopy was employed as the reference standard for evaluating device performance. Of 2,988 eligible patients, microscopy showed that 547 (18%) had P. falciparum, 658 (22%) had P. vivax, 2 (0.07%) had P. malariae, and 1,750 (59%) were negative for Plasmodium. Mixed infections (P. falciparum and P. vivax) were identified in 31 patients (1%). The overall sensitivity of ParaSight F for P. falciparum was 95%. When stratified by magnitude of parasitemia (no. of asexual parasites per microliter of whole blood), sensitivities were 83% (>0 to 500 parasites/microl), 87% (501 to 1,000/microl), 98% (1,001 to 5,000/microl), and 98% (>5,000/microl). Device specificity was 86%.
Figures
References
-
- Aron J L. Malaria epidemiology and detectability. Trans R Soc Med Hyg. 1982;76:595–601. - PubMed
-
- Bain B J, Chiodini P L, England J M, Bailey J W. The laboratory diagnosis of malaria. The Malaria Working Party of The General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 1997;19:165–170. - PubMed
-
- Banchongaksorn T, Prajakwong S, Rooney W, Vickers P. Operational trial of ParaSight-F (dipstick) in the diagnosis of falciparum malaria at the primary health care level. Southeast Asian J Trop Med Public Health. 1997;28:243–246. - PubMed
-
- Barat L, Chipipa J, Kolczak M, Sukwa T. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am J Trop Med Hyg. 1999;60:1024–1030. - PubMed
-
- Beadle C, Long G W, Weiss W R, McElroy P D, Maret S M, Oloo A J, Hoffman S L. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

