New insights into the role of cytokines in asthma
- PMID: 11477111
- PMCID: PMC1731485
- DOI: 10.1136/jcp.54.8.577
New insights into the role of cytokines in asthma
Abstract
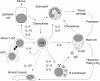
Asthma is a triad of intermittent airway obstruction, bronchial smooth muscle cell hyperreactivity to bronchoconstrictors, and chronic bronchial inflammation. From an aetiological standpoint, asthma is a heterogeneous disease, but often appears as a form of immediate hypersensitivity. Many patients with asthma have other manifestations of atopy, such as rhinitis or eczema. Even among non-atopic patients with asthma, the pathophysiology of airway constriction is similar, raising the hypothesis that alternative mechanisms of mast cell degranulation may underlie the disease. The primary inflammatory lesion of asthma consists of accumulation of CD4(+) T helper type 2 (TH2) lymphocytes and eosinophils in the airway mucosa. TH2 cells orchestrate the asthmatic inflammation through the secretion of a series of cytokines, particularly interleukin 4 (IL-4), IL-13, IL-5, and IL-9. IL-4 is the major factor regulating IgE production by B cells, and is required for optimal TH2 differentiation. However, blocking IL-4 is not sufficient to inhibit the development of asthma in experimental models. In contrast, inhibition of IL-13, another TH2 cytokine whose signal transduction pathway overlaps with that of IL-4, completely blocks airway hyperreactivity in mouse asthma models. IL-5 is a key factor for eosinophilia and could therefore be responsible for some of the tissue damage seen in chronic asthma. IL-9 has pleiotropic activities on allergic mediators such as mast cells, eosinophils, B cells and epithelial cells, and might be a good target for therapeutic interventions. Finally, chemokines, which can be produced by many cell types from inflamed lungs, play a major role in recruiting the mediators of asthmatic inflammation. Genetic studies have demonstrated that multiple genes are involved in asthma. Several genome wide screens point to chromosome 5q31--33 as a major susceptibility locus for asthma and high IgE values. This region includes a cluster of cytokine genes, and genes encoding IL-3, IL-4, IL-5, IL-9, IL-13, granulocyte macrophage colony stimulating factor, and the beta chain of IL-12. Interestingly, for some of these cytokines, a linkage was also established between asthma and their receptor. Another susceptibility locus has been mapped on chromosome 12 in a region that contains other potential candidate cytokine genes, including the gene encoding interferon gamma, the prototypical TH1 cytokine with inhibitory activities for TH2 lymphocytes. Taken together, both experimental and genetic studies point to TH2 cytokines, such as IL-4, IL-13, IL-5, and IL-9, as important targets for therapeutic applications in patients with asthma.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
