Epstein-Barr virus infection in paediatric liver transplant recipients: detection of the virus in post-transplant tonsillectomy specimens
- PMID: 11477143
- PMCID: PMC1187079
- DOI: 10.1136/mp.54.4.264
Epstein-Barr virus infection in paediatric liver transplant recipients: detection of the virus in post-transplant tonsillectomy specimens
Abstract
Aims: Post-transplant lymphoproliferative disease (PTLD) is an important and serious complication in transplant patients. Recent studies have suggested that quantitative assessment of Epstein-Barr virus (EBV) infection in transplant patients might help to identify those at risk of developing PTLD. Therefore, tonsils from paediatric liver transplant recipients were studied for evidence of EBV infection.
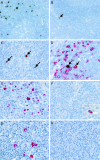
Methods: Tonsils were studied by in situ hybridisation for the detection of the small EBV encoded nuclear RNAs (EBERs). The phenotype of EBV infected cells was determined by double labelling in situ hybridisation and immunohistochemistry. The expression of viral latent and lytic antigens was determined by immunohistochemistry. Tonsils from patients without known immune defects were studied as controls.
Results: Tonsils from transplant patients showed pronounced follicular hyperplasia and minor paracortical hyperplasia. In situ hybridisation revealed variable numbers of EBV infected B cells in the tonsils from transplant patients (range, 2-1000/0.5 cm(2); mean, 434/0.5 cm(2); median, 105/0.5 cm(2)). Lower numbers were detected in the control tonsils (range, 1-200/0.5 cm(2); mean, 47/0.5 cm(2); median, 9/0.5 cm(2)). The latent membrane protein 1 (LMP1) of EBV was not detected and there were only rare cells in two cases showing expression of the EBV encoded nuclear antigen 2 (EBNA2). There was no evidence of lytic infection. None of the patients developed PTLD within a follow up period of up to five years.
Conclusions: These data indicate that tonsillar enlargement in paediatric liver transplant patients does not necessarily imply a diagnosis of PTLD. Furthermore, the presence of increased numbers of EBV infected cells in tonsils from liver transplant recipients by itself does not indicate an increased risk of developing PTLD.
Figures
References
-
- Nalesnik MA. Clinical and pathological features of post-transplant lymphoproliferative disorders (PTLD). Springer Semin Immunopathol 1998;20:325–42. - PubMed
-
- Lattyak BV, Rosenthal P, Mudge C, et al. Posttransplant lymphoproliferative disorder presenting in the head and neck. Laryngoscope 1998;108:1195–8. - PubMed
-
- Knowles DM, Cesarman E, Chadburn A, et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 1995;85:552–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
