Functional analysis of the four DNA binding domains of replication protein A. The role of RPA2 in ssDNA binding
- PMID: 11479296
- PMCID: PMC2796477
- DOI: 10.1074/jbc.M104386200
Functional analysis of the four DNA binding domains of replication protein A. The role of RPA2 in ssDNA binding
Abstract
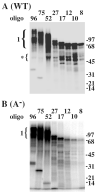
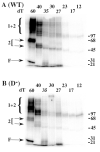
Replication Protein A (RPA), the heterotrimeric single-stranded DNA (ssDNA)-binding protein of eukaryotes, contains four ssDNA binding domains (DBDs) within its two largest subunits, RPA1 and RPA2. We analyzed the contribution of the four DBDs to ssDNA binding affinity by assaying recombinant yeast RPA in which a single DBD (A, B, C, or D) was inactive. Inactivation was accomplished by mutating the two conserved aromatic stacking residues present in each DBD. Mutation of domain A had the most severe effect and eliminated binding to a short substrate such as (dT)12. RPA containing mutations in DBDs B and C bound to substrates (dT)12, 17, and 23 but with reduced affinity compared with wild type RPA. Mutation of DBD-D had little or no effect on the binding of RPA to these substrates. However, mutations in domain D did affect the binding to oligonucleotides larger than 23 nucleotides (nt). Protein-DNA cross-linking indicated that DBD-A (in RPA1) is essential for RPA1 to interact efficiently with substrates of 12 nt or less and that DBD-D (RPA2) interacts efficiently with oligonucleotides of 27 nt or larger. The data support a sequential model of binding in which DBD-A is responsible for the initial interaction with ssDNA, that domains A, B, and C (RPA1) contact 12-23 nt of ssDNA, and that DBD-D (RPA2) is needed for RPA to interact with substrates that are 23-27 nt in length.
Figures





References
-
- Wold MS. Annu. Rev. Biochem. 1997;66:61–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

