ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor
- PMID: 11481474
- PMCID: PMC55377
- DOI: 10.1073/pnas.161290898
ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor
Abstract
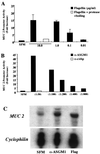
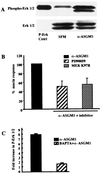
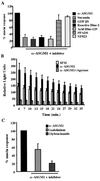
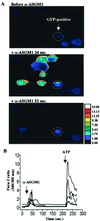
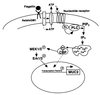
The flagella of the Gram-negative bacterium Pseudomonas aeruginosa serve not only for motility but also to bind bacteria to the host cell glycolipid asialoGM1 (ASGM1) through the protein flagellin. This interaction triggers defensive responses in host cells. How this response occurs is unclear because ASGM1 lacks transmembrane and cytoplasmic domains and there is little information about the downstream effectors that connect ASGM1 ligation to the initiation of host defense responses. Here, we show that ASGM1 ligation promotes ATP release from the host cell, followed by autocrine activation of a nucleotide receptor. This response links ASGM1 to cytoplasmic signaling molecules and results in activation of phospholipase C, Ca(2+) mobilization, phosphorylation of a mitogen-activated protein kinase (Erk 1/2), and activation of mucin transcription. These results indicate that bacterial interaction with host cells can trigger autocrine nucleotide signaling and suggest that agents affecting nucleotide receptors may modulate host responses to bacteria.
Figures


References
-
- Shapiro L. Cell. 1995;80:525–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

