Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions
- PMID: 11483497
- PMCID: PMC149144
- DOI: 10.1093/emboj/20.15.3938
Novel modular domain PB1 recognizes PC motif to mediate functional protein-protein interactions
Abstract
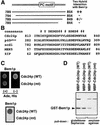
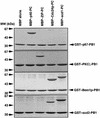
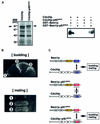
Modular domains mediating specific protein-protein interactions play central roles in the formation of complex regulatory networks to execute various cellular activities. Here we identify a novel domain PB1 in the budding yeast protein Bem1p, which functions in polarity establishment, and mammalian p67(phox), which activates the microbicidal phagocyte NADPH oxidase. Each of these specifically recognizes an evolutionarily conserved PC motif to interact directly with Cdc24p (an essential protein for cell polarization) and p40(phox) (a component of the signaling complex for the oxidase), respectively. Swapping the PB1 domain of Bem1p with that of p67(phox), which abolishes its interaction with Cdc24p, confers on cells temperature- sensitive growth and a bilateral mating defect. These phenotypes are suppressed by a mutant Cdc24p harboring the PC motif-containing region of p40(phox), which restores the interaction with the altered Bem1p. This domain-swapping experiment demonstrates that Bem1p function requires interaction with Cdc24p, in which the PB1 domain and the PC motif participate as responsible modules.
Figures





References
-
- Ago T., Nunoi,H., Ito,T. and Sumimoto,H. (1999) Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox: triple replacement of serines 303, 304, 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47phox, thereby activating the oxidase. J. Biol. Chem., 274, 33644–33653. - PubMed
-
- Butty A.-C., Pryciak,P.M., Huang,L.S., Herskowitz,I. and Peter,M. (1998) The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science, 282, 1511–1516. - PubMed
-
- Chang E.C., Barr,M., Wang,Y., Jung,V., Xu,H.-P. and Wigler,M.H. (1994) Cooperative interaction of S.pombe proteins required for mating and morphogenesis. Cell, 79, 131–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

