NEDD8 recruits E2-ubiquitin to SCF E3 ligase
- PMID: 11483504
- PMCID: PMC149148
- DOI: 10.1093/emboj/20.15.4003
NEDD8 recruits E2-ubiquitin to SCF E3 ligase
Abstract
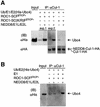
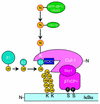
NEDD8/Rub1 is a ubiquitin (Ub)-like post-translational modifier that is covalently linked to cullin (Cul)-family proteins in a manner analogous to ubiquitylation. NEDD8 is known to enhance the ubiquitylating activity of the SCF complex (composed of Skp1, Cul-1, ROC1 and F-box protein), but the mechanistic role is largely unknown. Using an in vitro reconstituted system, we report here that NEDD8 modification of Cul-1 enhances recruitment of Ub-conjugating enzyme Ubc4 (E2) to the SCF complex (E3). This recruitment requires thioester linkage of Ub to Ubc4. Our findings indicate that the NEDD8-modifying system accelerates the formation of the E2-E3 complex, which stimulates protein polyubiquitylation.
Figures
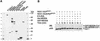
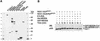
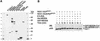





References
-
- Baldwin A.S. (1996) The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol., 14, 649–681. - PubMed
-
- Chen Y., McPhie,D.L., Hirschberg,J. and Neve,R.L. (2000) The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S–M checkpoint and causes apoptosis in neurons. J. Biol. Chem., 275, 8929–8935. - PubMed
-
- Deshaies R.J. (1999) SCF and Cullin/RING-H2-based ubiquitin-ligases. Annu. Rev. Cell. Dev. Biol., 15, 435–467. - PubMed
-
- Finco T.S. and Baldwin,A.S. (1995) Mechanistic aspects of NF-κB regulation: the emerging role of phosphorylation and proteolysis. Immunity, 3, 253–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

