Processing of 20S pre-rRNA to 18S ribosomal RNA in yeast requires Rrp10p, an essential non-ribosomal cytoplasmic protein
- PMID: 11483523
- PMCID: PMC149176
- DOI: 10.1093/emboj/20.15.4204
Processing of 20S pre-rRNA to 18S ribosomal RNA in yeast requires Rrp10p, an essential non-ribosomal cytoplasmic protein
Abstract
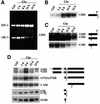
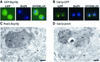
Numerous non-ribosomal trans-acting factors involved in pre-ribosomal RNA processing have been characterized, but none of them is specifically required for the last cytoplasmic steps of 18S rRNA maturation. Here we demonstrate that Rio1p/Rrp10p is such a factor. Previous studies showed that the RIO1 gene is essential for cell viability and conserved from archaebacteria to man. We isolated a RIO1 mutant in a screen for mutations synthetically lethal with a mutant allele of GAR1, an essential gene required for 18S rRNA production and rRNA pseudouridylation. We show that RIO1 encodes a cytoplasmic non-ribosomal protein, and that depletion of Rio1p blocks 18S rRNA production leading to 20S pre-rRNA accumulation. In situ hybridization reveals that, in Rio1p depleted cells, 20S pre-rRNA localizes in the cytoplasm, demonstrating that its accumulation is not due to an export defect. This strongly suggests that Rio1p is involved in the cytoplasmic cleavage of 20S pre-rRNA at site D, producing mature 18S rRNA. Thus, Rio1p has been renamed Rrp10p (ribosomal RNA processing #10). Rio1p/Rrp10p is the first non-ribosomal factor characterized specifically required for 20S pre-rRNA processing.
Figures




References
-
- Anaya P., Evans,S.C., Dai,C., Lozano,G. and May,G.S. (1998) Isolation of the Aspergillus nidulans sudD gene and its human homologue. Gene, 211, 323–329. - PubMed
-
- Angermayr M. and Bandlow,W. (1997) The type of basal promoter determines the regulated or constitutive mode of transcription in the common control region of the yeast gene pair GCY1/RIO1. J. Biol. Chem., 272, 31630–31635. - PubMed
-
- Bennetzen J.L. and Hall,B.D. (1982) Codon selection in yeast. J. Biol. Chem., 257, 3026–3031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

