Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit
- PMID: 11483700
- PMCID: PMC2278752
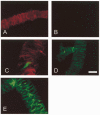
- DOI: 10.1111/j.1469-7793.2001.00691.x
Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit
Abstract
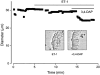
1. In this study we investigated the expression and function of the K(V)alpha1 subfamily of voltage-gated K(+) channels in terminal arterioles from rabbit cerebral circulation. 2. K(+) current was measured from smooth muscle cells within intact freshly isolated arteriolar fragments. Current activated on depolarisation positive of about -45 mV and a large fraction of this current was blocked by 3,4-diaminopyridine (3,4-DAP) or 4-aminopyridine (4-AP), inhibitors of K(V) channels. Expression of cRNA encoding K(V)1.6 in Xenopus oocytes also generated a 4-AP-sensitive K(+) current with a threshold for activation near -45 mV. 3. Immunofluorescence labelling revealed K(V)1.2 to be specifically localised to endothelial cells, and K(V)1.5 and K(V)1.6 to plasma membranes of smooth muscle cells. 4. K(V) channel current in arteriolar fragments was blocked by correolide (which is specific for the K(V)alpha1 family of K(V) channels) but was resistant to recombinant agitoxin-2 (rAgTX2; which inhibits K(V)1.6 but not K(V)1.5). Heterologously expressed K(V)2.1 was resistant to correolide, and K(V)1.6 was blocked by rAgTX2. 5. Arterioles that were mildly preconstricted and depolarised by 0.1-0.3 nM endothelin-1 constricted further in response to 3,4-DAP, 4-AP or correolide, but not to rAgTX2. 6. We suggest that K(V)alpha1 channels are expressed in smooth muscle cells of terminal arterioles, underlie a major part of the voltage-dependent K(+) current, and have a physiological function to oppose vasoconstriction. K(V)alpha1 complexes without K(V)1.5 appear to be uncommon.
Figures




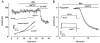
References
-
- Ahn DS, Hume JR. pH regulation of voltage-dependent K+ channels in canine pulmonary arterial smooth muscle cells. Pflügers Archiv. 1997;433:758–765. - PubMed
-
- Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, KV1. 5 and KV2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. Journal of Clinical Investigation. 1998;101:2319–2330. - PMC - PubMed
-
- Beech DJ. Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacology and Therapeutics. 1997;73:91–119. - PubMed
-
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

