Drastic reduction of the slow gate of human muscle chloride channel (ClC-1) by mutation C277S
- PMID: 11483705
- PMCID: PMC2278749
- DOI: 10.1111/j.1469-7793.2001.00745.x
Drastic reduction of the slow gate of human muscle chloride channel (ClC-1) by mutation C277S
Abstract
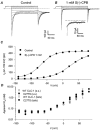
1. Single channel measurements suggest that the human muscle chloride channel ClC-1 presumably has a double barrelled structure, with a fast single protopore gate and a slow common pore gate similar to that of ClC-0, the chloride channel from Torpedo. The single point mutation C212S has been shown to abolish the slow gating of ClC-0 locking the slow gate in the open state. In order to test the hypothesis that the slow gating process found in ClC-1 corresponds to the well characterised slow gate found in ClC-0 we investigated the gating effects in ClC-1 of the homologous mutation corresponding to C212S, C277S. 2. We found that the mutation C277S strongly reduced the slow component of macroscopic gating relaxations at negative and at positive voltages. 3. Time constants of the fast gating relaxations were not affected by the mutation but the minimal open probability of the fast gate at negative voltages was slightly reduced to 0.08 compared with the WT value of 0.22. 4. Additionally, we characterised the block of WT ClC-1 and mutant C277S by the S(-) enantiomer of CPB (2-(p-chlorophenoxy) butyric acid), and found that the block is practically unaffected by the mutation suggesting that CPB does not interact with the slow gate of ClC-1. 5. We conclude that the slow and fast gating processes of ClC-1, respectively, reflect the slow common pore gate and the single protopore gate of the double-barrelled ClC-1 channel.
Figures



References
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. - PubMed
-
- Jentsch TJ, Steinmeyer K, Schwarz G. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 1990;348:510–514. - PubMed
-
- Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik K-H, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

