Differential expression and regulation of AE2 anion exchanger subtypes in rabbit parietal and mucous cells
- PMID: 11483713
- PMCID: PMC2278731
- DOI: 10.1111/j.1469-7793.2001.00837.x
Differential expression and regulation of AE2 anion exchanger subtypes in rabbit parietal and mucous cells
Abstract
1. The anion exchanger isoform 2 (AE2) gene encodes three subtypes (AE2a, b and c), which have different N-termini and tissue distributions. AE2 is expressed at high levels in the stomach, where it is thought to mediate basolateral base exit during acid production. The present study investigated if the three AE2 subtypes are differentially expressed and regulated in different cell types within the gastric mucosa. 2. The cloning strategy to obtain rabbit AE2a, b and c cDNAs combined genomic PCR and RT-PCR based on primers deduced from the rat sequences. Semiquantitative RT-PCR using homologous primers revealed much higher AE2 mRNA expression in rabbit parietal cells (PCs) than in mucous cells (MCs). The subtype expression pattern was AE2b >> AE2c > or = AE2a in PCs and AE2a >AE2b >> AE2c in MCs. Sequence analysis revealed the presence of a highly conserved protein kinase C (PKC) consensus sequence in the AE2a alternative N-terminus. 3. Maximal Cl(-)-HCO(3)(-) exchange rates, measured fluorometrically in BCECF-loaded cultured gastric cells, were much higher in PCs than MCs. PKC activation by phorbol ester stimulated maximal Cl(-)-HCO(3)(-) exchange rates in MCs but not in PCs, whereas forskolin had no effect in each cell type. 4. In summary, rabbit PCs and MCs, which originate from the same gastric stem cell population, display a completely different AE2 subtype expression pattern. Therefore, AE2 subtype expression is not organ specific but cell type specific. The different regulation of anion exchange in parietal and mucous cells suggests that AE2 subtypes may be differentially regulated.
Figures

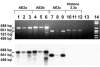
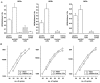

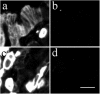



Similar articles
-
Inefficient chronic activation of parietal cells in Ae2a,b(-/-) mice.Am J Pathol. 2006 Jul;169(1):165-76. doi: 10.2353/ajpath.2006.051096. Am J Pathol. 2006. PMID: 16816370 Free PMC article.
-
Three 5'-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat.Ann N Y Acad Sci. 2000;915:81-91. doi: 10.1111/j.1749-6632.2000.tb05226.x. Ann N Y Acad Sci. 2000. PMID: 11193604 Review.
-
Three N-terminal variants of the AE2 Cl-/HCO3- exchanger are encoded by mRNAs transcribed from alternative promoters.J Biol Chem. 1996 Mar 29;271(13):7835-43. doi: 10.1074/jbc.271.13.7835. J Biol Chem. 1996. PMID: 8631828
-
Identification of the full-length AE2 (AE2a) isoform as the Golgi-associated anion exchanger in fibroblasts.J Histochem Cytochem. 2001 Feb;49(2):259-69. doi: 10.1177/002215540104900213. J Histochem Cytochem. 2001. PMID: 11156694
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
Cited by
-
cAMP-dependent and cholinergic regulation of the electrogenic intestinal/pancreatic Na+/HCO3- cotransporter pNBC1 in human embryonic kidney (HEK293) cells.BMC Cell Biol. 2008 Dec 22;9:70. doi: 10.1186/1471-2121-9-70. BMC Cell Biol. 2008. PMID: 19102757 Free PMC article.
-
KCNQ1 is the luminal K+ recycling channel during stimulation of gastric acid secretion.J Physiol. 2009 Aug 1;587(Pt 15):3955-65. doi: 10.1113/jphysiol.2009.173302. Epub 2009 Jun 2. J Physiol. 2009. PMID: 19491250 Free PMC article.
-
Molecular Characterization of Anion Exchanger 2 in Litopenaeus vannamei and Its Role in Nitrite Stress.Int J Mol Sci. 2025 Jan 23;26(3):964. doi: 10.3390/ijms26030964. Int J Mol Sci. 2025. PMID: 39940733 Free PMC article.
-
Pathophysiological role of ion channels and transporters in gastrointestinal mucosal diseases.Cell Mol Life Sci. 2021 Dec;78(24):8109-8125. doi: 10.1007/s00018-021-04011-5. Epub 2021 Nov 15. Cell Mol Life Sci. 2021. PMID: 34778915 Free PMC article. Review.
-
Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux.Acta Physiol (Oxf). 2018 Apr;222(4):e12923. doi: 10.1111/apha.12923. Epub 2017 Oct 19. Acta Physiol (Oxf). 2018. PMID: 28748627 Free PMC article.
References
-
- Alper SL. The band 3-related AE anion exchanger gene family. Cellular Physiology and Biochemistry. 1994;4:265–281.
-
- Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. American Journal of Physiology. 1999;40:G321–332. - PubMed
-
- Bachmann O, Sonnentag Th, Siegel W-K, Lamprecht G, Weichert A, Gregor M, Seidler U. Different secretagogues activate different Na+/H+ exchanger isoforms in rabbit parietal cells. American Journal of Physiology. 1998;275:G185–193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

