The envelope glycoprotein of friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk
- PMID: 11483734
- PMCID: PMC115033
- DOI: 10.1128/jvi.75.17.7893-7903.2001
The envelope glycoprotein of friend spleen focus-forming virus covalently interacts with and constitutively activates a truncated form of the receptor tyrosine kinase Stk
Abstract
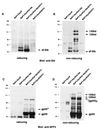
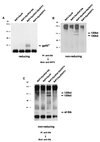
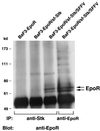
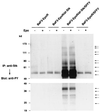
The Friend spleen focus-forming virus (SFFV) encodes a unique envelope glycoprotein, gp55, which allows erythroid cells to proliferate and differentiate in the absence of erythropoietin (Epo). SFFV gp55 has been shown to interact with the Epo receptor complex, causing constitutive activation of various signal-transducing molecules. When injected into adult mice, SFFV induces a rapid erythroleukemia, with susceptibility being determined by the host gene Fv-2, which was recently shown to be identical to the gene encoding the receptor tyrosine kinase Stk/Ron. Susceptible, but not resistant, mice encode not only full-length Stk but also a truncated form of the kinase, sf-Stk, which may mediate the biological effects of SFFV infection. To determine whether expression of SFFV gp55 leads to the activation of sf-Stk, we expressed sf-Stk, with or without SFFV gp55, in hematopoietic cells expressing the Epo receptor. Our data indicate that sf-Stk interacts with SFFV gp55 as well as gp55(P), the biologically active form of the viral glycoprotein, forming disulfide-linked complexes. This covalent interaction, as well as noncovalent interactions with SFFV gp55, results in constitutive tyrosine phosphorylation of sf-Stk and its association with multiple tyrosine-phosphorylated signal-transducing molecules. In contrast, neither Epo stimulation in the absence of SFFV gp55 expression nor expression of a mutant of SFFV that cannot interact with sf-Stk was able to induce tyrosine phosphorylation of sf-Stk or its association with any signal-transducing molecules. Covalent interaction of sf-Stk with SFFV gp55 and constitutive tyrosine phosphorylation of sf-Stk can also be detected in an erythroleukemia cell line derived from an SFFV-infected mouse. Our results suggest that SFFV gp55 may mediate its biological effects in vivo by interacting with and activating a truncated form of the receptor tyrosine kinase Stk.
Figures




References
-
- Behringer R R, Dewey M J. Cellular site and mode of Fv-2 gene action. Cell. 1985;40:441–447. - PubMed
-
- Bondurant M C, Koury M J, Krantz S B. The Fv-2 gene controls induction of erythroid burst formation by Friend virus infection in vitro: studies of growth regulators and viral replication. J Gen Virol. 1985;66:83–93. - PubMed
-
- Casadevall N, Lacombe C, Muller O, Gisselbrecht S, Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells) J Biol Chem. 1991;266:16015–16020. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

