Specific interaction of a novel foamy virus Env leader protein with the N-terminal Gag domain
- PMID: 11483744
- PMCID: PMC115043
- DOI: 10.1128/jvi.75.17.7995-8007.2001
Specific interaction of a novel foamy virus Env leader protein with the N-terminal Gag domain
Abstract
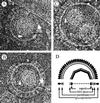
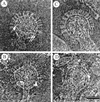
Cryoelectron micrographs of purified human foamy virus (HFV) and feline foamy virus (FFV) particles revealed distinct radial arrangements of Gag proteins. The capsids were surrounded by an internal Gag layer that in turn was surrounded by, and separated from, the viral membrane. The width of this layer was about 8 nm for HFV and 3.8 nm for FFV. This difference in width is assumed to reflect the different sizes of the HFV and FFV MA domains: the HFV MA domain is about 130 residues longer than that of FFV. The distances between the MA layer and the edge of the capsid were identical in different particle classes. In contrast, only particles with a distended envelope displayed an invariant, close spacing between the MA layer and the Env membrane which was absent in the majority of particles. This indicates a specific interaction between MA and Env at an unknown step of morphogenesis. This observation was supported by surface plasmon resonance studies. The purified N-terminal domain of FFV Gag specifically interacted with synthetic peptides and a defined protein domain derived from the N-terminal Env leader protein. The specificity of this interaction was demonstrated by using peptides varying in the conserved Trp residues that are known to be required for HFV budding. The interaction with Gag required residues within the novel virion-associated FFV Env leader protein of about 16.5 kDa.
Figures





Similar articles
-
Mutagenesis of N-terminal residues of feline foamy virus Gag reveals entirely distinct functions during capsid formation, particle assembly, Gag processing and budding.Retrovirology. 2016 Aug 22;13(1):57. doi: 10.1186/s12977-016-0291-8. Retrovirology. 2016. PMID: 27549192 Free PMC article.
-
N-terminally myristoylated feline foamy virus Gag allows Env-independent budding of sub-viral particles.Viruses. 2011 Nov;3(11):2223-37. doi: 10.3390/v3112223. Epub 2011 Nov 14. Viruses. 2011. PMID: 22163342 Free PMC article.
-
Human foamy virus capsid formation requires an interaction domain in the N terminus of Gag.J Virol. 2001 May;75(9):4367-75. doi: 10.1128/JVI.75.9.4367-4375.2001. J Virol. 2001. PMID: 11287585 Free PMC article.
-
Particle assembly and genome packaging.Curr Top Microbiol Immunol. 2003;277:89-110. doi: 10.1007/978-3-642-55701-9_4. Curr Top Microbiol Immunol. 2003. PMID: 12908769 Review.
-
The foamy virus envelope glycoproteins.Curr Top Microbiol Immunol. 2003;277:111-29. doi: 10.1007/978-3-642-55701-9_5. Curr Top Microbiol Immunol. 2003. PMID: 12908770 Review.
Cited by
-
Foamy virus envelope glycoprotein is sufficient for particle budding and release.J Virol. 2003 Feb;77(4):2338-48. doi: 10.1128/jvi.77.4.2338-2348.2003. J Virol. 2003. PMID: 12551971 Free PMC article.
-
Evolution of foamy viruses: the most ancient of all retroviruses.Viruses. 2013 Sep 25;5(10):2349-74. doi: 10.3390/v5102349. Viruses. 2013. PMID: 24072062 Free PMC article. Review.
-
An N-terminal domain helical motif of Prototype Foamy Virus Gag with dual functions essential for particle egress and viral infectivity.Retrovirology. 2013 Apr 25;10:45. doi: 10.1186/1742-4690-10-45. Retrovirology. 2013. PMID: 23618494 Free PMC article.
-
Mutagenesis of N-terminal residues of feline foamy virus Gag reveals entirely distinct functions during capsid formation, particle assembly, Gag processing and budding.Retrovirology. 2016 Aug 22;13(1):57. doi: 10.1186/s12977-016-0291-8. Retrovirology. 2016. PMID: 27549192 Free PMC article.
-
Discovery of prosimian and afrotherian foamy viruses and potential cross species transmissions amidst stable and ancient mammalian co-evolution.Retrovirology. 2014 Aug 4;11:61. doi: 10.1186/1742-4690-11-61. Retrovirology. 2014. PMID: 25091111 Free PMC article.
References
-
- Alke A, Schwantes A, Zemba M, Flügel R M, Löchelt M. Characterization of the humoral immune response and virus replication in cats experimentally infected with feline foamy virus. Virology. 2000;275:170–176. - PubMed
-
- Bodem J, Zemba M, Flügel R M. Nuclear localization of the functional Bel 1 transactivator but not of the gag proteins of the feline foamy virus. Virology. 1998;251:22–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

