Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection
- PMID: 11483761
- PMCID: PMC115060
- DOI: 10.1128/jvi.75.17.8158-8165.2001
Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection
Abstract
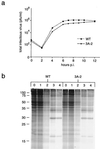
During viral infections, the host secretory pathway is crucial for both innate and acquired immune responses. For example, the export of most proinflammatory and antiviral cytokines, which recruit lymphocytes and initiate antiviral defenses, requires traffic through the host secretory pathway. To investigate potential effects of the known inhibition of cellular protein secretion during poliovirus infection on pathogenesis, cytokine secretion from cells infected with wild-type virus and with 3A-2, a mutant virus carrying an insertion in viral protein 3A which renders the virus defective in the inhibition of protein secretion, was tested. We show here that cells infected with 3A-2 mutant virus secrete greater amounts of cytokines interleukin-6 (IL-6), IL-8, and beta interferon than cells infected with wild-type poliovirus. Increased cytokine secretion from the mutant-infected cells can be attributed to the reduced inhibition of host protein secretion, because no significant differences between 3A-2- and wild-type-infected cells were observed in the inhibition of viral growth, host cell translation, or the ability of wild-type- or 3A-2-infected cells to support the transcriptional induction of beta interferon mRNA. We surmise that the wild-type function of 3A in inhibiting ER-to-Golgi traffic is not required for viral replication in tissue culture but, by altering the amount of secreted cytokines, could have substantial effects on pathogenesis within an infected host. The global inhibition of protein secretion by poliovirus may reflect a general mechanism by which pathogens that do not require a functional protein secretory apparatus can reduce the native immune response and inflammation associated with infection.
Figures



References
-
- Bazzigher L, Pavlovic J, Haller O, Staeheli P. Mx genes show weaker primary response to virus than other interferon-regulated genes. Virology. 1992;186:154–160. - PubMed
-
- Belsham G J, Jackson R J. Translation initiation on picornavirus RNA. In: Sonenberg N, Hershey J W B, Mathews M B, editors. Translational control of gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. pp. 869–900.
-
- Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

