Correlated neuronal activity and the flow of neural information
- PMID: 11483997
- PMCID: PMC2868968
- DOI: 10.1038/35086012
Correlated neuronal activity and the flow of neural information
Abstract
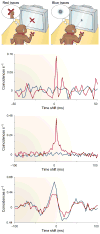
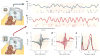
For years we have known that cortical neurons collectively have synchronous or oscillatory patterns of activity, the frequencies and temporal dynamics of which are associated with distinct behavioural states. Although the function of these oscillations has remained obscure, recent experimental and theoretical results indicate that correlated fluctuations might be important for cortical processes, such as attention, that control the flow of information in the brain.
Figures


References
-
- Seidemann E, Zohary U, Newsome WT. Temporal gating of neural signals during performance of a visual discrimination task. Nature. 1998;394:72–75. A rare inquiry into how neural signals are gated. Microstimulation pulses were applied in the middle temporal visual cortex (MT) during a visual motion discrimination task. Their effect depended critically on the timing of the pulses relative to the time of stimulus presentation. - PubMed
-
- Barlow JS. The Electroencephalogram: its Patterns and Origins. MIT Press; Cambridge, Massachusetts: 1993.
-
- Borbèly AA, Hayaishi O, Sejnowski TJ, Altman JS. Human Frontier Science Program. Strasbourg: 2000. The Regulation of Sleep.
-
- Destexhe A, Sejnowski TJ. Why do we sleep? Brain Res. 2000;886:208–223. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

