The perifollicular and marginal zones of the human splenic white pulp : do fibroblasts guide lymphocyte immigration?
- PMID: 11485909
- PMCID: PMC1850570
- DOI: 10.1016/S0002-9440(10)61722-1
The perifollicular and marginal zones of the human splenic white pulp : do fibroblasts guide lymphocyte immigration?
Abstract
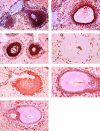
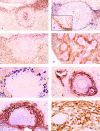
We investigate the white pulp compartments of 73 human spleens and demonstrate that there are several microanatomical peculiarities in man that do not occur in rats or mice. Humans lack a marginal sinus separating the marginal zone (MZ) from the follicles or the follicular mantle zone. The MZ is divided into an inner and an outer compartment by a special type of fibroblasts. An additional compartment, termed the perifollicular zone, is present between the follicular MZ and the red pulp. The perifollicular zone contains sheathed capillaries and blood-filled spaces without endothelial lining. In the perifollicular zone, in the outer MZ, and in the T cell zone fibroblasts of an unusual phenotype occur. These cells stain for the adhesion molecules MAdCAM-1, VCAM-1 (CD106), and VAP-1; the Thy-1 (CD90) molecule; smooth muscle alpha-actin and smooth muscle myosin; cytokeratin 18; and thrombomodulin (CD141). They are, however, negative for the peripheral node addressin, the cutaneous lymphocyte antigen, CD34, PECAM-1 (CD31), and P- and E-selectin (CD62P and CD62E). In the MZ the fibroblasts are often tightly associated with CD4-positive T lymphocytes, whereas CD8-positive cells are almost absent. Our findings lead to the hypothesis, that recirculating CD4-positive T lymphocytes enter the human splenic white pulp from the open circulation of the perifollicular zone without crossing an endothelium. Specialized fibroblasts may attract these T cells and guide them into the periarteriolar T cell area.
Figures
References
-
- Van Krieken JHJM, te Velde J: Immunohistology of the human spleen: an inventory of the localization of lymphocyte subpopulations. Histopathology 1986, 10:285-294 - PubMed
-
- Grogan TM, Jolley CS, Rangel CS: Immunoarchitecture of the human spleen. Lymphology 1983, 16:72-82 - PubMed
-
- Grogan TM, Rangel CS, Richter LC, Wirt DP, Villar HV: Further delineation of the immuno-architecture of the human spleen. Lymphology 1984, 17:61-68 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

