Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice
- PMID: 11485910
- PMCID: PMC1850533
- DOI: 10.1016/S0002-9440(10)61723-3
Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice
Abstract
Receptor for advanced glycation end-products (RAGE), and two of its ligands, AGE and EN-RAGEs (members of the S100/calgranulin family of pro-inflammatory cytokines), display enhanced expression in slowly resolving full-thickness excisional wounds developed in genetically diabetic db+/db+ mice. We tested the concept that blockade of RAGE, using soluble(s) RAGE, the extracellular ligand-binding domain of the receptor, would enhance wound closure in these animals. Administration of sRAGE accelerated the development of appropriately limited inflammatory cell infiltration and activation in wound foci. In parallel with accelerated wound closure at later times, blockade of RAGE suppressed levels of cytokines; tumor necrosis factor-alpha; interleukin-6; and matrix metalloproteinases-2, -3, and -9. In addition, generation of thick, well-vascularized granulation tissue was enhanced, in parallel with increased levels of platelet-derived growth factor-B and vascular endothelial growth factor. These findings identify a central role for RAGE in disordered wound healing associated with diabetes, and suggest that blockade of this receptor might represent a targeted strategy to restore effective wound repair in this disorder.
Figures
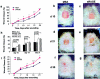
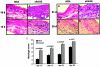
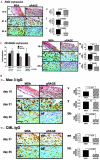
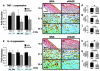

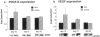

Comment in
-
Inflammation in nonhealing diabetic wounds: the space-time continuum does matter.Am J Pathol. 2001 Aug;159(2):399-403. doi: 10.1016/S0002-9440(10)61709-9. Am J Pathol. 2001. PMID: 11485896 Free PMC article. Review. No abstract available.
References
-
- Goodson WH, Hunt TK: Wound healing and the diabetic patient. Surg Gynecol Obstet 1979, 149:690-698 - PubMed
-
- Morain WD, Colen LB: Wound healing in diabetes mellitus. Clin Plast Surg 1990, 17:493-501 - PubMed
-
- Goodson WH, Hunt TK: Studies of wound healing in experimental diabetes. J Surg Res 1977, 22:221-227 - PubMed
-
- Schaffer CJ, Nanney LB: Cell biology of wound healing. Int Rev Cytol 1996, 169:151-181 - PubMed
-
- Ehrlich HP: The physiology of wound healing. A summary of normal and abnormal wound healing processes. Adv Wound Care 1998, 11:326-328 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

