Role of peroxisome proliferator-activated receptor gamma and its ligands in non-neoplastic and neoplastic human urothelial cells
- PMID: 11485917
- PMCID: PMC1850548
- DOI: 10.1016/s0002-9440(10)61730-0
Role of peroxisome proliferator-activated receptor gamma and its ligands in non-neoplastic and neoplastic human urothelial cells
Abstract
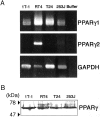
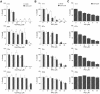
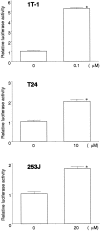
Peroxisome proliferator-activated receptor gamma (PPARgamma) is a member of the nuclear receptor superfamily of ligand-activated transcription factors and is expressed in several types of tissue. Although PPARgamma reportedly is expressed in normal urothelium, its function is unknown. We examined the expression of PPARgamma in normal urothelium and bladder cancer in an attempt to assess its functional role. Immunohistochemical staining revealed normal urothelium to express PPARgamma uniformly. All low-grade carcinomas were positive either diffusely or focally, whereas staining was primarily focal or absent in high-grade carcinomas. A nonneoplastic urothelial cell line (1T-1), a low-grade (RT4) carcinoma cell line, and two high-grade (T24 and 253J) carcinoma cell lines in culture expressed PPARgamma mRNA and protein. Luciferase assay indicated that PPARgamma was functional. PPARgamma ligands (15-deoxy-Delta(12,14)-prostaglandin J(2), troglitazone and pioglitazone) suppressed the growth of nonneoplastic and neoplastic urothelial cells in a dose-dependent manner. However, neoplastic cells were more resistant than were nonneoplastic cells. Failure to express PPARgamma or ineffective transcriptional activity may be some of the mechanisms responsible for resistance to the inhibitory action of PPARgamma ligands.
Figures

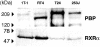
References
-
- Zhu Y, Alvares K, Huang Q, Rao MS, Reddy JK: Cloning of a new member of the peroxisome proliferator-activated receptor gene family from mouse liver. J Biol Chem 1993, 268:26817-26820 - PubMed
-
- Tontonoz P, Hu E, Spiegelman BM: Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79:1147-1156 - PubMed
-
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W: Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rats. Endocrinology 1996, 137:354-366 - PubMed
-
- Guan Y, Zhang Y, Davis L, Breyer MD: Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol 1997, 273:F1013-F1022 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

