Renal cholesterol accumulation: a durable response after acute and subacute renal insults
- PMID: 11485932
- PMCID: PMC1850565
- DOI: 10.1016/S0002-9440(10)61745-2
Renal cholesterol accumulation: a durable response after acute and subacute renal insults
Abstract
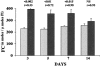
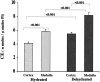
Proximal tubular cholesterol levels rise within 18 hours of diverse forms of acute renal tubular injury (eg, myoglobinuria, ischemia/reperfusion, urinary tract obstruction). These increments serve to protect against further bouts of tubular attack (so-called "acquired cytoresistance"). Whether these cholesterol increments are merely transitory, or persist into the maintenance phase of acute renal failure (ARF), has not been previously defined. Furthermore, whether subacute/insidious tubular injury [eg, cyclosporine A (CSA), tacrolimus toxicity], nontubular injury (eg, acute glomerulonephritis), or physiological stress (eg, mild dehydration) impact renal cholesterol homeostasis have not been addressed. This study sought to resolve these issues. Male CD-1 mice were subjected to glycerol-induced ARF. Renal cortical-free cholesterol (FC) and cholesterol ester (CE) levels were determined 3, 5, 7, or 14 days later, and the values contrasted to prevailing blood-urea nitrogen concentrations. The impact of 40 minutes of unilateral renal ischemia plus reflow (3 to 6 days) on mouse cortical FC/CE content was also assessed. Additionally, FC/CE levels were measured in rat renal cortex either 10 days after CSA or tacrolimus therapy, or 48 hours after induction of nephrotoxic serum nephritis. Finally, the impact of overnight dehydration on mouse renal cortical/medullary FC/CE profiles was determined. Compared to sham-treated animals, glycerol, CSA, tacrolimus, ischemia-reperfusion, and nephrotoxic serum each induced dramatic CE +/- FC elevations, rising as much as 10x control values. In the glycerol model, striking correlations (r </= 0.99) between FC/CE and blood-urea nitrogen levels were observed. The FC/CE increases were specific to damaged kidney (glycerol did not raise hepatic FC/CE; unilateral renal ischemia did not alter contralateral renal FC/CE levels). Overnight dehydration raised renal CE levels, most notably in the medulla.
Conclusions: FC/CE accumulation is a hallmark of the maintenance phase of ischemic and nephrotoxic ARF, and can reflect its severity. That cholesterol accumulation can result from glomerular injury and dehydration suggests that it is a generic renal stress response, with potential relevance extending beyond just the phenomenon of acquired cytoresistance.
Figures
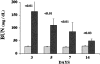
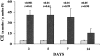

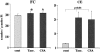

References
-
- Honda N, Hishida A, Ikuma K, Yonemura K: Acquired resistance to acute renal failure. Kidney Int 1987, 31:1233-1238 - PubMed
-
- Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA: Acquired resistance to acute renal failure: possible role of heme oxygenase and ferritin. Lab Invest 1995, 72:474-483 - PubMed
-
- Hayes JM, Boonshaft B, Maher JF, O’Connell JMB, Schriener GE: Resistance to glycerol-induced acute renal failure. Nephron 1970, 7:155-164 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

