In vitro reconstitution of the end replication problem
- PMID: 11486015
- PMCID: PMC87295
- DOI: 10.1128/MCB.21.17.5753-5766.2001
In vitro reconstitution of the end replication problem
Abstract
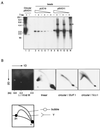
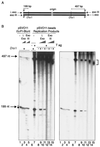
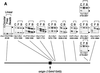
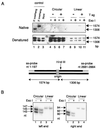
The end replication problem hypothesis proposes that the ends of linear DNA cannot be replicated completely during lagging strand DNA synthesis. Although the idea has been widely accepted for explaining telomere attrition during cell proliferation, it has never been directly demonstrated. In order to take a biochemical approach to understand how linear DNA ends are replicated, we have established a novel in vitro linear simian virus 40 DNA replication system. In this system, terminally biotin-labeled linear DNAs are conjugated to avidin-coated beads and subjected to replication reactions. Linear DNA was efficiently replicated under optimized conditions, and replication products that had replicated using the original DNA templates were specifically analyzed by purifying bead-bound replication products. By exploiting this system, we showed that while the leading strand is completely synthesized to the end, lagging strand synthesis is gradually halted in the terminal approximately 500-bp region, leaving 3' overhangs. This result is consistent with observations in telomerase-negative mammalian cells and formally demonstrates the end replication problem. This study provides a basis for studying the details of telomere replication.
Figures



References
-
- Blasco M A, Lee H W, Hande M P, Samper E, Lansdorp P M, DePinho R A, Greider C W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. - PubMed
-
- Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
-
- Braguglia D, Heun P, Pasero P, Duncker B P, Gasser S M. Semi-conservative replication in yeast nuclear extracts requires Dna2 helicase and supercoiled template. J Mol Biol. 1998;281:631–649. - PubMed
-
- Brewer B J, Fangman W L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987;51:463–471. - PubMed
-
- Brush G S, Kelly T J, Stillman B. Identification of eukaryotic DNA replication proteins using simian virus 40 in vitro replication system. Methods Enzymol. 1995;262:522–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
