Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis
- PMID: 11486033
- PMCID: PMC87313
- DOI: 10.1128/MCB.21.17.5946-5957.2001
Identification of receptor and heparin binding sites in fibroblast growth factor 4 by structure-based mutagenesis
Abstract
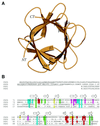
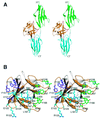
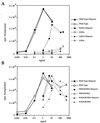
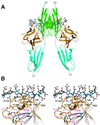
Fibroblast growth factors (FGFs) comprise a large family of multifunctional, heparin-binding polypeptides that show diverse patterns of interaction with a family of receptors (FGFR1 to -4) that are subject to alternative splicing. FGFR binding specificity is an essential mechanism in the regulation of FGF signaling and is achieved through primary sequence differences among FGFs and FGFRs and through usage of two alternative exons, IIIc and IIIb, for the second half of immunoglobulin-like domain 3 (D3) in FGFRs. While FGF4 binds and activates the IIIc splice forms of FGFR1 to -3 at comparable levels, it shows little activity towards the IIIb splice forms of FGFR1 to -3 as well as towards FGFR4. To begin to explore the structural determinants for this differential affinity, we determined the crystal structure of FGF4 at a 1.8-A resolution. FGF4 adopts a beta-trefoil fold similar to other FGFs. To identify potential receptor and heparin binding sites in FGF4, a ternary FGF4-FGFR1-heparin model was constructed by superimposing the FGF4 structure onto FGF2 in the FGF2-FGFR1-heparin structure. Mutation of several key residues in FGF4, observed to interact with FGFR1 or with heparin in the model, produced ligands with reduced receptor binding and concomitant low mitogenic potential. Based on the modeling and mutational data, we propose that FGF4, like FGF2, but unlike FGF1, engages the betaC'-betaE loop in D3 and thus can differentiate between the IIIc and IIIb splice isoforms of FGFRs for binding. Moreover, we show that FGF4 needs to interact with both the 2-O- and 6-O-sulfates in heparin to exert its optimal biological activity.
Figures

References
-
- Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. - PubMed
-
- Bruenger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nigles M, Pannu N S, Read R J, Rice L M, Simonson T, Warren G L. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr Sect D. 1998;54:905–921. - PubMed
-
- Delli Bovi P, Curatola A M, Kern F G, Greco A, Ittmann M, Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987;50:729–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
