Voltage-dependent acceleration of Ca(v)1.2 channel current decay by (+)- and (-)-isradipine
- PMID: 11487504
- PMCID: PMC1572885
- DOI: 10.1038/sj.bjp.0704181
Voltage-dependent acceleration of Ca(v)1.2 channel current decay by (+)- and (-)-isradipine
Abstract
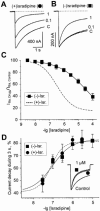
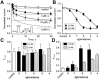
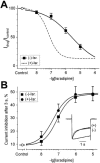
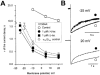
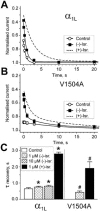
Inhibition of Ca(v)1.2 by antagonist 1,4 dihydropyridines (DHPs) is associated with a drug-induced acceleration of the calcium (Ca(2+)) channel current decay. This feature is contradictorily interpreted as open channel block or as drug-induced inactivation. To elucidate the underlying molecular mechanism we investigated the effects of (+)- and (-)-isradipine on Ca(v)1.2 inactivation gating at different membrane potentials. alpha(1)1.2 Constructs were expressed together with alpha(2)-delta- and beta(1a)- subunits in Xenopus oocytes and drug-induced changes in barium current (I(Ba)) kinetics analysed with the two microelectrode voltage clamp technique. To study isradipine effects on I(Ba) decay without contamination by intrinsic inactivation we expressed a mutant (V1504A) lacking fast voltage-dependent inactivation. At a subthreshold potential of -30 mV a 200-times higher concentration of (-)-isradipine was required to induce a comparable amount of inactivation as by (+)-isradipine. At +20 mV the two enantiomers were equally efficient in accelerating the I(Ba) decay. Faster recovery from (-)- than from (+)-isradipine-induced inactivation at -80 mV in a Ca(v)1.2 construct (tau((-)-isr.(Cav1.2))=0.74 s<tau((+)-isr.(Cav1.2))=2.85 s) and even more rapid recovery of V1504A (tau((-)-isr.(V1504A))=0.39 s<tau((+)-isr.(V1504A))=1.98 s) indicated that drug-induced determinants and determinants of intrinsic inactivation (V1504) stabilize the DHP-induced channel conformation in an additive manner. In the voltage range between -25 and 20 mV where the channels inactivate predominantly from the open state the (+)- and (-)-isradipine-induced acceleration of the I(Ba) decay in V1504A displayed similar voltage-dependence as intrinsic fast inactivation of Ca(v)1.2. Our data suggest that the isradipine-induced acceleration of the Ca(v)1.2 current decay reflects enhanced fast voltage-dependent inactivation and not open channel block.
Figures

References
-
- BERJUKOW S., GAPP F., ACZEL S., SINNEGGER M.J., MITTERDORFER J., GLOSSMANN H., HERING S. Sequence differences between alpha1C and alpha1S Ca2+ channel subunits reveal structural determinants of a guarded and modulated benzothiazepine receptor. J. Biol. Chem. 1999;274:6154–6160. - PubMed
-
- BERJUKOW S., MARKSTEINER R., GAPP F., SINNEGGER M.J., HERING S. Molecular mechanism of calcium channel block by isradipine. Role of a drug induced inactivated channel conformation. J. Biol. Chem. 2000;275:22114–22120. - PubMed
-
- BREHM P., ECKERT R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978;202:1203–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

