Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism
- PMID: 11487506
- PMCID: PMC1572861
- DOI: 10.1038/sj.bjp.0704152
Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism
Abstract
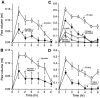
Thrombin, generated in the circulation during injury, cleaves proteinase-activated receptor 1 (PAR1) to stimulate plasma extravasation and granulocyte infiltration. However, the mechanism of thrombin-induced inflammation in intact tissues is unknown. We hypothesized that thrombin cleaves PAR1 on sensory nerves to release substance P (SP), which interacts with the neurokinin 1 receptor (NK1R) on endothelial cells to cause plasma extravasation. PAR1 was detected in small diameter neurons known to contain SP in rat dorsal root ganglia by immunohistochemistry and in situ hybridization. Thrombin and the PAR1 agonist TFLLR-NH(2) (TF-NH(2)) increased [Ca(2+)](i) >50% of cultured neurons (EC(50)s 24 mu ml(-1) and 1.9 microM, respectively), assessed using Fura-2 AM. The PAR1 agonist completely desensitized responses to thrombin, indicating that thrombin stimulates neurons through PAR1. Injection of TF-NH(2) into the rat paw stimulated a marked and sustained oedema. An NK1R antagonist and ablation of sensory nerves with capsaicin inhibited oedema by 44% at 1 h and completely by 5 h. In wild-type but not PAR1(-/-) mice, TF-NH(2) stimulated Evans blue extravasation in the bladder, oesophagus, stomach, intestine and pancreas by 2 - 8 fold. Extravasation in the bladder, oesophagus and stomach was abolished by an NK1R antagonist. Thus, thrombin cleaves PAR1 on primary spinal afferent neurons to release SP, which activates the NK1R on endothelial cells to stimulate gap formation, extravasation of plasma proteins, and oedema. In intact tissues, neurogenic mechanisms are predominantly responsible for PAR1-induced oedema.
Figures







References
-
- BROKAW J.J., WHITE G.W. Differential effects of phosphoramidon and captopril on NK1 receptor-mediated plasma extravasation in the rat trachea. Agents Actions. 1994;42:34–39. - PubMed
-
- CHEN Y.H., POUYSSEGUR J., COURTNEIDGE S.A., VAN OBBERGHEN-SCHILLING E. Activation of Src family kinase activity by the G protein-coupled thrombin receptor in growth-responsive fibroblasts. J. Biol. Chem. 1994;269:27372–27377. - PubMed
-
- CHEUNG W.M., D'ANDREA M.R., ANDRADE-GORDON P., DAMIANO B.P. Altered vascular injury responses in mice deficient in protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 1999;19:3014–3024. - PubMed
-
- CIRINO G., BUCCI M., CICALA C., NAPOLI C. Inflammation-coagulation network: are serine protease receptors the knot. Trends Pharmacol. Sci. 2000;21:170–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

