A dominant negative inhibitor of the Egr family of transcription regulatory factors suppresses cerebellar granule cell apoptosis by blocking c-Jun activation
- PMID: 11487612
- PMCID: PMC6763154
- DOI: 10.1523/JNEUROSCI.21-16-05893.2001
A dominant negative inhibitor of the Egr family of transcription regulatory factors suppresses cerebellar granule cell apoptosis by blocking c-Jun activation
Abstract
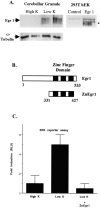
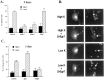
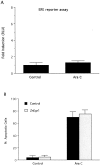
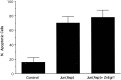
To investigate the role of the Egr family of transcription regulatory factors in neuronal apoptosis, we examined the effect of a dominant negative Egr inhibitor construct in a well characterized in vitro paradigm, cerebellar granule cell death induced by withdrawal of depolarizing concentrations of extracellular potassium. We found that this apoptotic stimulus increases the activity of a reporter gene driven by the Egr response element and that a dominant negative inhibitor of Egr-mediated transcription blocks granule cell apoptosis. In contrast, apoptosis of immature granule cells induced by cytosine arabinoside is not inhibited by the Egr inhibitor construct. Because activation of c-Jun is an essential step in granule cell death induced by potassium deprivation, but not cytosine arabinoside, we asked whether the Egr inhibitor acts by influencing c-Jun activation or its ability to induce apoptosis. We found that the Egr inhibitor does not block the ability of a constitutively active c-Jun construct to induce apoptosis in these cells but does suppress activation of c-Jun-mediated transcription induced by lowering extracellular potassium concentration. Furthermore, the Egr inhibitor blocks the ability of MEKK1 [mitogen-activated protein kinase (MAPK) kinase kinase 1], an upstream kinase capable of stimulating the JNK (c-Jun N-terminal protein kinase)-c-Jun pathway, to induce apoptosis and activate c-Jun. Together, these studies indicate that the Egr family of transcription factors plays a critical role in neuronal apoptosis and identify c-Jun activation as an important downstream target of the Egr family in this process.
Figures



Similar articles
-
A dominant negative Egr inhibitor blocks nerve growth factor-induced neurite outgrowth by suppressing c-Jun activation: role of an Egr/c-Jun complex.J Neurosci. 2002 May 15;22(10):3845-54. doi: 10.1523/JNEUROSCI.22-10-03845.2002. J Neurosci. 2002. PMID: 12019303 Free PMC article.
-
Blockade of NGF-induced neurite outgrowth by a dominant-negative inhibitor of the egr family of transcription regulatory factors.J Neurosci. 2001 Jan 1;21(1):45-52. doi: 10.1523/JNEUROSCI.21-01-00045.2001. J Neurosci. 2001. PMID: 11150318 Free PMC article.
-
Insulin-like growth factor-I blocks Bcl-2 interacting mediator of cell death (Bim) induction and intrinsic death signaling in cerebellar granule neurons.J Neurosci. 2002 Nov 1;22(21):9287-97. doi: 10.1523/JNEUROSCI.22-21-09287.2002. J Neurosci. 2002. PMID: 12417654 Free PMC article.
-
Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors.Curr Cancer Drug Targets. 2004 Feb;4(1):43-52. doi: 10.2174/1568009043481704. Curr Cancer Drug Targets. 2004. PMID: 14965266 Review.
-
Targeting the JNK pathway for therapeutic benefit in CNS disease.Curr Drug Targets CNS Neurol Disord. 2002 Feb;1(1):31-49. doi: 10.2174/1568007023339472. Curr Drug Targets CNS Neurol Disord. 2002. PMID: 12769633 Review.
Cited by
-
A dominant negative Egr inhibitor blocks nerve growth factor-induced neurite outgrowth by suppressing c-Jun activation: role of an Egr/c-Jun complex.J Neurosci. 2002 May 15;22(10):3845-54. doi: 10.1523/JNEUROSCI.22-10-03845.2002. J Neurosci. 2002. PMID: 12019303 Free PMC article.
-
Opposing roles for ATF2 and c-Fos in c-Jun-mediated neuronal apoptosis.Mol Cell Biol. 2009 May;29(9):2431-42. doi: 10.1128/MCB.01344-08. Epub 2009 Mar 2. Mol Cell Biol. 2009. PMID: 19255142 Free PMC article.
-
Early Growth Response Gene-1 Suppresses Foot-and-Mouth Disease Virus Replication by Enhancing Type I Interferon Pathway Signal Transduction.Front Microbiol. 2018 Sep 27;9:2326. doi: 10.3389/fmicb.2018.02326. eCollection 2018. Front Microbiol. 2018. PMID: 30319594 Free PMC article.
-
An early response transcription factor, Egr-1, enhances insulin resistance in type 2 diabetes with chronic hyperinsulinism.J Biol Chem. 2011 Apr 22;286(16):14508-15. doi: 10.1074/jbc.M110.190165. Epub 2011 Feb 14. J Biol Chem. 2011. PMID: 21321112 Free PMC article.
-
Modulation of protein S and growth arrest specific 6 protein signaling inhibits pancreatic cancer cell survival and proliferation.Oncol Rep. 2020 Oct;44(4):1322-1332. doi: 10.3892/or.2020.7689. Epub 2020 Jul 15. Oncol Rep. 2020. PMID: 32945517 Free PMC article.
References
-
- Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. - PubMed
-
- Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet. 1999;21:326–329. - PubMed
-
- Catania MV, Copani A, Calogero A, Ragonese GI, Condorelli DF, Nicoletti F. An enhanced expression of the immediate early gene, Egr-1, is associated with neuronal apoptosis in culture. Neuroscience. 1999;91:1529–1538. - PubMed
-
- Chapman NR, Perkins ND. Inhibition of the RelA(p65) NF-kappaB subunit by Egr-1. J Biol Chem. 2000;275:4719–4724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
