Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons
- PMID: 11487631
- PMCID: PMC6763192
- DOI: 10.1523/JNEUROSCI.21-16-06077.2001
Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons
Abstract
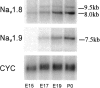
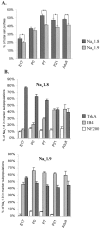
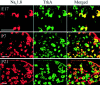
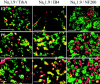
The development of neuronal excitability involves the coordinated expression of different voltage-gated ion channels. We have characterized the expression of two sensory neuron-specific tetrodotoxin-resistant sodium channel alpha subunits, Na(v)1. (SNS/PN3) and Na(v)1.9 (SNS2/NaN), in developing rat lumbar dorsal root ganglia (DRGs). Expression of both Na(v)1.8 and Na(v)1.9 increases with age, beginning at embryonic day (E) 15 and E17, respectively, and reaching adult levels by postnatal day 7. Their distribution is restricted mainly to those subpopulations of primary sensory neurons in developing and adult DRGs that give rise to unmyelinated C-fibers (neurofilament 200 negative). Na(v)1.8 is expressed in a higher proportion of neuronal profiles than Na(v)1.9 at all stages during development, as in the adult. At E17, almost all Na(v)1.8-expressing neurons also express the high-affinity NGF receptor TrkA, and only a small proportion bind to IB4, a marker for c-ret-expressing (glial-derived neurotrophic factor-responsive) neurons. Because IB4 binding neurons differentiate from TrkA neurons in the postnatal period, the proportion of Na(v)1.8 cells that bind to IB4 increases, in parallel with a decrease in the proportion of Na(v)1.8-TrkA co-expressing cells. In contrast, an equal number of Na(v)1.9 cells bind IB4 and TrkA in embryonic life. The differential expression of Na(v)1.8 and Na(v)1.9 in late embryonic development, with their distinctive kinetic properties, may contribute to the development of spontaneous and stimulus-evoked excitability in small diameter primary sensory neurons in the perinatal period and the activity-dependent changes in differentiation they produce.
Figures

References
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
-
- Altman J, Bayer SA. The development of the rat spinal cord. Adv Anat Embryol Cell Biol. 1984;85:1–164. - PubMed
-
- Alvares D, Fitzgerald M. Building blocks of pain: the regulation of key molecules in spinal sensory neurones during development and following peripheral axotomy. Pain. 1999;6:S71–85. - PubMed
-
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
