Cyclic nucleotide-mediated regulation of hippocampal mossy fiber development: a target-specific guidance
- PMID: 11487641
- PMCID: PMC6763174
- DOI: 10.1523/JNEUROSCI.21-16-06181.2001
Cyclic nucleotide-mediated regulation of hippocampal mossy fiber development: a target-specific guidance
Abstract
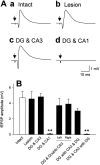
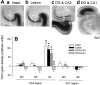
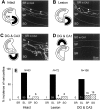
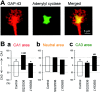
The mossy fibers (MFs) arising from dentate granule cells project primarily onto a narrow segment of the proximal dendrites of hippocampal CA3 pyramidal cells. The mechanisms underlying this specific MF target selection are not fully understood. To investigate the cellular basis for development of the stereotyped MF trajectories, we have arranged the fascia dentata and hippocampal Ammon's horn tissues in diverse topographical patterns in organotypic explant coculture systems. Here we show that cyclic nucleotide signaling pathways regulate the MF pathfinding. When the dentate gyrus explants were ectopically placed facing the CA3 stratum oriens of hippocampal slices, MFs crossed the border between cocultures and reached their appropriate target area in the Ammon's horn, as assessed by membrane tracer labeling, Timm staining, electrophysiological recording of synaptic responses, and optical analyses using a voltage-sensitive dye. This lamina-specific MF innervation was disrupted by pharmacological blockade of cGMP pathway. Similar apposition of the dentate grafts near the CA1 region of host slices rarely resulted in MF ingrowth into the Ammon's horn. Under blockade of cAMP pathway, however, the MFs were capable of making allopatric synapses with CA1 neurons. These data were further supported by the pharmacological data obtained from granule cells dispersed over hippocampal slice cultures. Thus, our findings suggest that the stereotyped MF extension is mediated by at least two distinct factors, i.e., an attractant derived from the CA3 region and a repellent from the CA1 region. These factors may be regulated differently by cAMP and cGMP signaling pathways.
Figures






References
-
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet. 1998;19:289–291. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. - PubMed
-
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
-
- Bonhoeffer F, Huf J. Recognition of cell types by axonal growth cones in vitro. Nature. 1980;288:162–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
