Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis
- PMID: 11487645
- PMCID: PMC6763166
- DOI: 10.1523/JNEUROSCI.21-16-06221.2001
Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis
Abstract
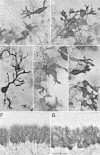
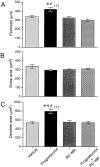
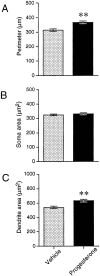
De novo steroidogenesis from cholesterol is a conserved property of vertebrate brains, and such steroids synthesized de novo in the brain are called neurosteroids. The identification of neurosteroidogenic cells is essential to the understanding of the physiological role of neurosteroids in the brain. We have demonstrated recently that neuronal neurosteroidogenesis occurs in the brain and indicated that the Purkinje cell actively synthesizes several neurosteroids de novo from cholesterol in vertebrates. Interestingly, in the rat, this neuron actively synthesizes progesterone de novo from cholesterol only during neonatal life, when cerebellar cortical formation occurs most markedly. Therefore, in this study, the possible organizing actions of progesterone during cerebellar development have been examined. In vitro studies using cerebellar slice cultures from newborn rats showed that progesterone promotes dose-dependent dendritic outgrowth of Purkinje cells but dose not affect their somata. This effect was blocked by the anti-progestin RU 486 [mifepristone; 17beta-hydroxy-11beta-(4-methylaminophenyl)-17alpha-(1-propynyl) estra-4,9-dien-3 one-6-7]. In vivo administration of progesterone to pups further revealed an increase in the density of Purkinje spine synapses electron microscopically. In contrast to progesterone, there was no significant effect of 3alpha,5alpha-tetrahydroprogesterone, a progesterone metabolite, on Purkinje cell development. Reverse transcription-PCR-Southern and immunocytochemical analyses showed that intranuclear progesterone receptors were expressed in Purkinje cells. These results suggest that progesterone promotes both dendritic outgrowth and synaptogenesis in Purkinje cells through intranuclear receptor-mediated mechanisms during cerebellar development. Such organizing actions may contribute to the formation of the cerebellar neuronal circuit.
Figures





Similar articles
-
Dendritic spine formation in response to progesterone synthesized de novo in the developing Purkinje cell in rats.Neurosci Lett. 2002 Apr 5;322(2):111-5. doi: 10.1016/s0304-3940(02)00077-0. Neurosci Lett. 2002. PMID: 11958856
-
Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell.Endocrinology. 2003 Oct;144(10):4466-77. doi: 10.1210/en.2003-0307. Epub 2003 Jul 17. Endocrinology. 2003. PMID: 12960093
-
Biosynthesis and organizing action of neurosteroids in the developing Purkinje cell.Cerebellum. 2006;5(2):89-96. doi: 10.1080/14734220600697211. Cerebellum. 2006. PMID: 16818383 Review.
-
Kobayashi award: Discovery of cerebellar and pineal neurosteroids and their biological actions on the growth and survival of Purkinje cells during development (review).Gen Comp Endocrinol. 2019 Dec 1;284:113051. doi: 10.1016/j.ygcen.2018.10.014. Epub 2018 Oct 16. Gen Comp Endocrinol. 2019. PMID: 30339808 Review.
-
Progesterone biosynthesis and action in the developing neuron.Endocrinology. 2008 Jun;149(6):2757-61. doi: 10.1210/en.2007-1592. Epub 2008 Feb 28. Endocrinology. 2008. PMID: 18308850 Review.
Cited by
-
Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease.Essays Biochem. 2020 Sep 23;64(3):591-606. doi: 10.1042/EBC20200043. Essays Biochem. 2020. PMID: 32756865 Free PMC article. Review.
-
Imaging of tau deposits in adults with Niemann-Pick type C disease: a case-control study.Eur J Nucl Med Mol Imaging. 2019 May;46(5):1132-1138. doi: 10.1007/s00259-019-4273-7. Epub 2019 Jan 28. Eur J Nucl Med Mol Imaging. 2019. PMID: 30690666
-
Nuclear hormone receptors in demyelinating diseases.J Neuroendocrinol. 2022 Jul;34(7):e13171. doi: 10.1111/jne.13171. Epub 2022 Jun 22. J Neuroendocrinol. 2022. PMID: 35734821 Free PMC article. Review.
-
Neurosteroids in the Purkinje cell: biosynthesis, mode of action and functional significance.Mol Neurobiol. 2008 Apr-Jun;37(2-3):116-25. doi: 10.1007/s12035-008-8024-1. Epub 2008 Jun 3. Mol Neurobiol. 2008. PMID: 18521763 Review.
-
Sensorimotor Development in Rat Neonates Exposed to 17-α-Hydroxyprogesterone Caproate: A Progestin Used in Obstetrics.Neuroendocrinology. 2025;115(8):678-688. doi: 10.1159/000546356. Epub 2025 May 21. Neuroendocrinology. 2025. PMID: 40398404
References
-
- Akwa Y, Young J, Kabbadj K, Sancho MJ, Zucman D, Vourc'h C, Jung-Testas I, Hu ZY, Le Goascogne C, Jo DH, Corpéchot C, Simon P, Baulieu EE, Robel P. Neurosteroids: biosynthesis, metabolism and function of pregnenolone and dehydroepiandrosterone in the brain. J Steroid Biochem Mol Biol. 1991;40:71–81. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972a;145:353–398. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972b;145:399–464. - PubMed
-
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the CNS. Annu Rev Neurosci. 1984;7:413–442. - PubMed
-
- Baulieu EE. Neurosteroids: a new function in the brain. Biol Cell. 1991;71:3–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
