Increasing the number of synapses modifies olfactory perception in Drosophila
- PMID: 11487649
- PMCID: PMC6763191
- DOI: 10.1523/JNEUROSCI.21-16-06264.2001
Increasing the number of synapses modifies olfactory perception in Drosophila
Abstract
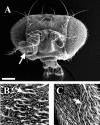
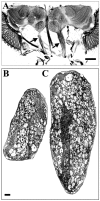
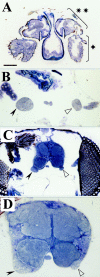
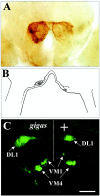
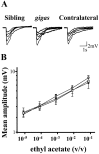
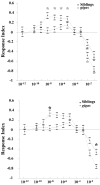
The Drosophila mutant gigas produces an enlargement of postmitotic cells caused by additional rounds of DNA replication. In neurons, the mutant cell establishes more synapses than normal. We have taken advantage of this feature to study the effect of synapse number on odorant perception. Mosaic adults were generated in which one antenna was homozygous for gigas, whereas the contralateral side served as an internal control. Morphological analysis indicates that the number and type of sensory afferents forming the mutant antenna, as well as their projection to the olfactory glomeruli, are normal. In contrast, the volume of identified glomeruli increases to a variable extent, and mutant sensory neurons branch profusely. The number of synapses, estimated in the ventral (V) glomerulus that receives ipsilateral afferents only, is increased twofold to threefold. Large-dense-core vesicle-containing terminals that probably modulate olfactory centers are identified in the V glomerulus. Their number and size are not modified by the mutant input. Sensory transduction, measured by electroantennograms, is normal in amplitude and kinetics. In odorant tests, however, the profile of the behavioral response to ethyl acetate shows attractive responses to concentrations to which sibling controls remain indifferent (10(-)8 and 10(-)7 v/v). In addition, the intensity of the response is augmented both at attractive and repulsive odorant concentrations with respect to that of controls. These results demonstrate that increased synapse number in the sensory neurons can modify the behavior of the organism, allowing a higher sensitivity of perception.
Figures

References
-
- Alcorta E. Characterization of the electroantennogram in Drosophila melanogaster and its use for identifying olfactory capture and transduction mutants. J Neurophysiol. 1991;65:702–714. - PubMed
-
- Anton S, Homberg U. Antennal lobe structure. In: Hansson BS, editor. Insect olfaction. Springer; Berlin: 1999. pp. 98–125.
-
- Ayer RK, Jr, Carlson J. Olfactory physiology in the Drosophila antenna and maxillary palp: acj6 distinguishes two classes of odorant pathways. J Neurobiol. 1992;23:965–982. - PubMed
-
- Ayyub C, Paranjape J, Rodrigues V, Siddiqi O. Genetics of olfactory behavior in Drosophila melanogaster. J Neurogenet. 1990;6:243–262. - PubMed
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Neurosci. 1993;55:397–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
