Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee
- PMID: 11487663
- PMCID: PMC6763189
- DOI: 10.1523/JNEUROSCI.21-16-06395.2001
Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee
Abstract
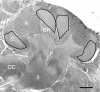
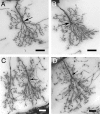
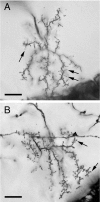
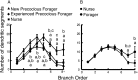
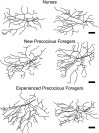
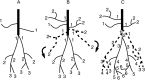
A worker honeybee performs tasks within the hive for approximately the first 3 weeks of adult life. After this time, it becomes a forager, flying repeatedly to collect food outside of the hive for the remainder of its 5-6 week life. Previous studies have shown that foragers have an increased volume of neuropil associated with the mushroom bodies, a brain region involved in learning, memory, and sensory integration. We report here that growth of the mushroom body neuropil in adult bees occurs throughout adult life and continues after bees begin to forage. Studies using Golgi impregnation asked whether the growth of the collar region of the mushroom body neuropil was a result of growth of the dendritic processes of the mushroom body intrinsic neurons, the Kenyon cells. Branching and length of dendrites in the collar region of the calyces were strongly correlated with worker age, but when age-matched bees were directly compared, those with foraging experience had longer, more branched dendrites than bees that had foraged less or not at all. The density of Kenyon cell dendritic spines remained constant regardless of age or behavioral state. Older and more experienced foragers therefore have a greater total number of dendritic spines in the mushroom body neuropil. Our findings indicate that, under natural conditions, the cytoarchitectural complexity of neurons in the mushroom bodies of adult honeybees increases as a function of increasing age, but that foraging experience promotes additional dendritic branching and growth.
Figures




Similar articles
-
Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees.J Neurobiol. 1995 Jan;26(1):130-44. doi: 10.1002/neu.480260111. J Neurobiol. 1995. PMID: 7714522
-
Muscarinic regulation of Kenyon cell dendritic arborizations in adult worker honey bees.Arthropod Struct Dev. 2011 Sep;40(5):409-19. doi: 10.1016/j.asd.2011.01.003. Epub 2011 Jan 22. Arthropod Struct Dev. 2011. PMID: 21262388 Free PMC article.
-
Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachilidae).Dev Neurobiol. 2008 Jan;68(1):73-82. doi: 10.1002/dneu.20574. Dev Neurobiol. 2008. PMID: 17918235
-
Juvenile hormone, behavioral maturation, and brain structure in the honey bee.Dev Neurosci. 1996;18(1-2):102-14. doi: 10.1159/000111474. Dev Neurosci. 1996. PMID: 8840089 Review.
-
Gamma-aminobutyric acid in the honey bee mushroom bodies - is inhibition the wellspring of plasticity?Curr Opin Insect Sci. 2024 Dec;66:101278. doi: 10.1016/j.cois.2024.101278. Epub 2024 Oct 5. Curr Opin Insect Sci. 2024. PMID: 39369905 Review.
Cited by
-
Nutritionally driven differential gene expression leads to heterochronic brain development in honeybee castes.PLoS One. 2013 May 30;8(5):e64815. doi: 10.1371/journal.pone.0064815. Print 2013. PLoS One. 2013. PMID: 23738002 Free PMC article.
-
Analysis of the Differentiation of Kenyon Cell Subtypes Using Three Mushroom Body-Preferential Genes during Metamorphosis in the Honeybee (Apis mellifera L.).PLoS One. 2016 Jun 28;11(6):e0157841. doi: 10.1371/journal.pone.0157841. eCollection 2016. PLoS One. 2016. PMID: 27351839 Free PMC article.
-
Age and rearing environment interact in the retention of early olfactory memories in honeybees.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008 Jul;194(7):629-40. doi: 10.1007/s00359-008-0337-z. Epub 2008 Apr 26. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008. PMID: 18438671
-
Extensive reorganization of behavior accompanies ontogeny of aggression in male flesh flies.PLoS One. 2014 Apr 8;9(4):e93196. doi: 10.1371/journal.pone.0093196. eCollection 2014. PLoS One. 2014. PMID: 24714439 Free PMC article.
-
Caste-biased patterns of brain investment in the subterranean termite Reticulitermes flavipes.iScience. 2024 May 22;27(6):110052. doi: 10.1016/j.isci.2024.110052. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883809 Free PMC article.
References
-
- Bertholf LM. The moults of the honeybee. J Econ Entomol. 1925;18:380–384.
-
- Brandon J, Coss R. Rapid dendritic spine shortening during one-trial learning: the honeybee's first orientation flight. Brain Res. 1982;252:51–61. - PubMed
-
- Bullock TH, Horridge GA. Structure and function in the nervous systems of invertebrates. Freeman; San Francisco: 1965.
-
- Burrows M. The neurobiology of an insect brain. Oxford UP; New York: 1996.
-
- Capaldi EA, Smith AD, Osborne J, Fahrbach SE, Farris SM, Reynolds DR, Edwards AS, Martin A, Robinson GE, Poppy GM, Riley JR. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature. 2000;403:537–540. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
