The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia
- PMID: 11487665
- PMCID: PMC6763168
- DOI: 10.1523/JNEUROSCI.21-16-06413.2001
The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia
Abstract
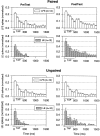
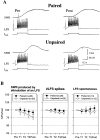
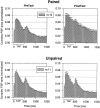
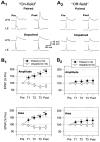
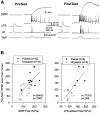
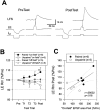
Plasticity at central synapses has long been thought to be the most likely mechanism for learning and memory, but testing that idea experimentally has proven to be difficult. For this reason, we have developed a simplified preparation of the Aplysia siphon withdrawal reflex that allows one to examine behavioral learning and memory while simultaneously monitoring synaptic connections between individual identified neurons in the CNS. We previously found that monosynaptic connections from LE siphon sensory neurons to LFS siphon motor neurons make a substantial contribution to the reflex in the siphon withdrawal preparation (Antonov et al., 1999a). We have now used that preparation to assess the contribution of various cellular mechanisms to classical conditioning of the reflex with a siphon tap conditioned stimulus (CS) and tail shock unconditioned stimulus (US). We find that, compared with unpaired training, paired training with the CS and US produces greater enhancement of siphon withdrawal and evoked firing of LFS neurons, greater facilitation of the complex PSP elicited in an LFS neuron by the siphon tap, and greater facilitation of the monosynaptic PSP elicited by stimulation of a single LE neuron. Moreover, the enhanced facilitation of monosynaptic LE-LFS PSPs is greater for LE neurons that fire during the siphon tap and correlates significantly with the enhancement of siphon withdrawal and evoked firing of the LFS neurons. These results provide the most direct evidence to date that activity-dependent plasticity at specific central synapses contributes to behavioral conditioning and support the idea that synaptic plasticity is a mechanism of learning and memory more generally.
Figures




References
-
- Antonov I, Antonova I, Hawkins RD. Activity-dependent facilitation of monosynaptic sensory neuron-motor neuron PSPs contributes to classical conditioning of the Aplysia siphon-withdrawal reflex in a simplified preparation. Soc Neurosci Abstr. 1999b;25:1129.
-
- Antonov I, Kandel ER, Hawkins RD. Contribution of pre- and postsynaptic mechanisms to activity-dependent facilitation during classical conditioning of the Aplysia siphon-withdrawal reflex. Soc Neurosci Abstr. 2000;26:1523.
-
- Bailey CH, Castellucci VF, Koester J, Kandel ER. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. J Neurophysiol. 1979;42:530–557. - PubMed
-
- Bao J-X, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275:969–973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
