Chattering and differential signal processing in identified motion-sensitive neurons of parallel visual pathways in the chick tectum
- PMID: 11487668
- PMCID: PMC6763139
- DOI: 10.1523/JNEUROSCI.21-16-06440.2001
Chattering and differential signal processing in identified motion-sensitive neurons of parallel visual pathways in the chick tectum
Abstract
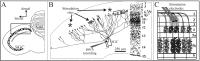
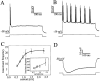
At least three identified cell types in the stratum griseum centrale (SGC) of the chick optic tectum mediate separate pathways from the retina to different subdivisions of the thalamic nucleus rotundus. Two of these, SGC type I and type II, constitute the major direct inputs to rotundal subdivisions that process various aspects of visual information, e.g., motion and luminance changes. Here, we examined the responses of these cell types to somatic current injection and synaptic input. We used a brain slice preparation of the chick tectum and applied whole-cell patch recordings, restricted electrical stimulation of dendritic endings, and subsequent labeling with biocytin. Type I neurons responded with regular sequences of bursts ("chattering") to depolarizing current injection. Electrical stimulation of retinal afferents evoked a sharp-onset EPSP/burst response that was blocked with CNQX. The sharp-onset EPSP/burst response to synaptic stimulation persisted when the soma was hyperpolarized, thus suggesting the presence of dendritic spike generation. In contrast, the type II neurons responded to depolarizing current injection solely with an irregular sequence of individual spikes. Electrical stimulation of retinal afferents led to slow and long-lasting EPSPs that gave rise to one or several action potentials. In conclusion, the morphological distinct SGC type I and II neurons also have different response properties to retinal inputs. This difference is likely to have functional significance for the differential processing of visual information in the separate pathways from the retina to different subdivisions of the thalamic nucleus rotundus.
Figures





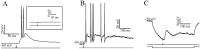
Similar articles
-
Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the tectofugal pathway.J Comp Neurol. 1998 Jul 6;396(3):399-414. J Comp Neurol. 1998. PMID: 9624592
-
Sparse spatial sampling for the computation of motion in multiple stages.Biol Cybern. 2006 Apr;94(4):276-87. doi: 10.1007/s00422-005-0046-4. Epub 2006 Jan 10. Biol Cybern. 2006. PMID: 16402243
-
Processing of motion stimuli by cells in the optic tectum of chickens.Neuroreport. 2015 Jul 8;26(10):578-82. doi: 10.1097/WNR.0000000000000391. Neuroreport. 2015. PMID: 26053699
-
Candidate molecular mechanisms for establishing cell identity in the developing retina.Dev Neurobiol. 2011 Dec;71(12):1258-72. doi: 10.1002/dneu.20926. Dev Neurobiol. 2011. PMID: 21630473 Free PMC article. Review.
-
The Visual Brain: Computing Through Multiscale Complexity.2016 May 3. In: Buzsáki G, Christen Y, editors. Micro-, Meso- and Macro-Dynamics of the Brain [Internet]. Cham (CH): Springer; 2016. 2016 May 3. In: Buzsáki G, Christen Y, editors. Micro-, Meso- and Macro-Dynamics of the Brain [Internet]. Cham (CH): Springer; 2016. PMID: 28590679 Free Books & Documents. Review.
Cited by
-
Mapping of the receptive fields in the optic tectum of chicken (Gallus gallus) using sparse noise.PLoS One. 2013 Apr 8;8(4):e60782. doi: 10.1371/journal.pone.0060782. Print 2013. PLoS One. 2013. PMID: 23593310 Free PMC article.
-
The mouse pulvinar nucleus: Organization of the tectorecipient zones.Vis Neurosci. 2017 Jan;34:E011. doi: 10.1017/S0952523817000050. Vis Neurosci. 2017. PMID: 28965504 Free PMC article. Review.
-
A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon.J Neurosci. 2007 Jul 25;27(30):8112-21. doi: 10.1523/JNEUROSCI.1420-07.2007. J Neurosci. 2007. PMID: 17652602 Free PMC article.
-
Evolution of the amniote pallium and the origins of mammalian neocortex.Ann N Y Acad Sci. 2011 Apr;1225:14-27. doi: 10.1111/j.1749-6632.2011.06006.x. Ann N Y Acad Sci. 2011. PMID: 21534989 Free PMC article. Review.
-
Ultrastructure of ipsilateral and contralateral tectopulvinar projections in the mouse.J Comp Neurol. 2022 May;530(7):1099-1111. doi: 10.1002/cne.25264. Epub 2021 Oct 24. J Comp Neurol. 2022. PMID: 34636423 Free PMC article.
References
-
- Angaut P, Reperant J. Fine structure of the optic fibre termination layers in the pigeon optic tectum: a Golgi and electron microscope study. Neuroscience. 1976;1:93–105. - PubMed
-
- Benowitz LI, Karten HJ. Organization of the tectofugal visual pathway in the pigeon: a retrograde transport study. J Comp Neurol. 1976;167:503–520. - PubMed
-
- Binns KE, Salt TE. Excitatory amino acid receptors participate in synaptic transmission of visual responses in the superficial layers of the cat superior colliculus. Eur J Neurosci. 1994;6:161–169. - PubMed
-
- Bravo H, Pettigrew JD. The distribution of neurons projecting from the retina and visual cortex to the thalamus and tectum opticum of the barn owl, Tyto alba, and the burrowing owl, Speotyto cunicularia. J Comp Neurol. 1981;199:419–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
