The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release
- PMID: 11487694
- PMCID: PMC139137
- DOI: 10.1105/tpc.010116
The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release
Abstract
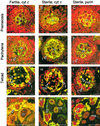
In mammals, mitochondria have been shown to play a key intermediary role in apoptosis, a morphologically distinct form of programmed cell death (PCD), for example, through the release of cytochrome c, which activates a proteolytic enzyme cascade, resulting in specific nuclear DNA degradation and cell death. In plants, PCD is a feature of normal development, including the penultimate stage of anther development, leading to dehiscence and pollen release. However, there is little evidence that plant mitochondria are involved in PCD. In a wide range of plant species, anther and/or pollen development is disrupted in a class of mutants termed CMS (for cytoplasmic male sterility), which is associated with mutations in the mitochondrial genome. On the basis of the manifestation of a number of morphological and biochemical markers of apoptosis, we have shown that the PET1-CMS cytoplasm in sunflower causes premature PCD of the tapetal cells, which then extends to other anther tissues. These features included cell condensation, oligonucleosomal cleavage of nuclear DNA, separation of chromatin into delineated masses, and initial persistence of mitochondria. In addition, immunocytochemical analysis revealed that cytochrome c was released partially from the mitochondria into the cytosol of tapetal cells before the gross morphological changes associated with PCD. The decrease in cytochrome c content in mitochondria isolated from male sterile florets preceded a decrease in the integrity of the outer mitochondrial membrane and respiratory control ratio. Our data suggest that plant mitochondria, like mammalian mitochondria, play a key role in the induction of PCD. The tissue-specific nature of the CMS phenotype is discussed with regard to cellular respiratory demand and PCD during normal anther development.
Figures







References
-
- Balk, J., Leaver, C.J., and McCabe, P.F. (1999). Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett. 463, 151–154. - PubMed
-
- Beers, E. (1997). Programmed cell death during plant growth and development. Cell Death Differ. 4, 649–661. - PubMed
-
- Bino, R., Suurs, L.C.J.M., De Hoop, S.J., Van der Neut, A., Van Went, J.L., and Van Marrewijk, G.A.M. (1986). Characterization of cytoplasmic male sterility in Petunia hybrida and Zea mays: Localization and activity of cytochrome c oxidase. Euphytica 35, 905–918.
-
- Cai, J., Yang, J., and Jones, D.P. (1998). Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta 1366, 139–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

