Dynamic recruitment of Cdc2 to specific microtubule structures during mitosis
- PMID: 11487703
- PMCID: PMC139136
- DOI: 10.1105/tpc.010109
Dynamic recruitment of Cdc2 to specific microtubule structures during mitosis
Abstract
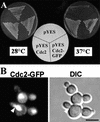
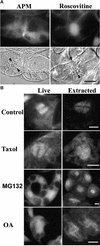
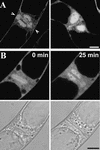
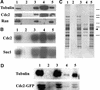
A-type cyclin-dependent kinases (CDKs), also known as cdc2, are central to the orderly progression of the cell cycle. We made a functional Green Fluorescent Protein (GFP) fusion with CDK-A (Cdc2-GFP) and followed its subcellular localization during the cell cycle in tobacco cells. During interphase, the Cdc2-GFP fusion protein was found in both the cytoplasm and the nucleus, where it was highly resistant to extraction. In premitotic cells, a bright and narrow equatorial band appeared on the cell surface, resembling the late preprophase band, which disintegrated within 10 min as followed by time-lapse images. Cdc2-GFP was not found on prophase spindles but left the chromatin soon after this stage and associated progressively with the metaphase spindle in a microtubule-dependent manner. Arresting cells in mitosis through the stabilization of microtubules by taxol further enhanced the spindle-localized pool of Cdc2-GFP. Toward the end of mitosis, Cdc2-GFP was found at the midzone of the anaphase spindle and phragmoplast; eventually, it became focused at the midline of these microtubule structures. In detergent-extracted cells, the Cdc2-GFP remained associated with mitotic structures. Retention on spindles was prevented by pretreatment with the CDK-specific inhibitor roscovitine and was enhanced by the protein phosphatase inhibitor okadaic acid. Furthermore, we demonstrate that both the endogenous CDK-A and Cdc2-GFP were cosedimented with taxol-stabilized plant microtubules from cell extracts and that Cdc2 activity was detected together with a fraction of polymerized tubulin. These data provide evidence that the A-type CDKs associate physically with mitotic structures in a microtubule-dependent manner and may be involved in regulating the behavior of specific microtubule arrays throughout mitosis.
Figures





References
-
- Alfa, C.E., Ducommun, B., Beach, D., and Hyams, J.S. (1990). Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature 347, 680–682. - PubMed
-
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179–189. - PubMed
-
- Ayaydin, F., Vissi, E., Meszaros, T., Miskolczi, P., Kovacs, I., Feher, A., Dombradi, V., Erdodi, F., Gergely, P., and Dudits, D. (2000). Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M tran-sition and early mitotic microtubule organisation in alfalfa. Plant J. 23, 85–96. - PubMed
-
- Barroso, C., Chan, J., Allan, V., Doonan, J., Hussey, P., and Lloyd, C. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: Characterization of a novel kinesin, DcKRP120–2. Plant J. 24, 859–868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

