Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus
- PMID: 11489871
- PMCID: PMC95394
- DOI: 10.1128/JB.183.17.5171-5179.2001
Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus
Abstract
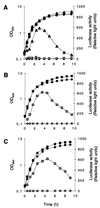
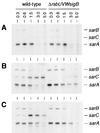
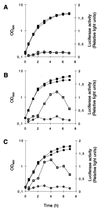
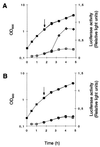
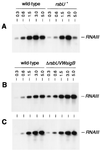
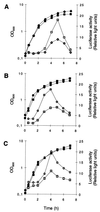
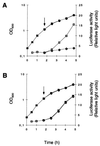
The growth phase-dependent activity profile of the alternate transcription factor sigma(B) and its effects on the expression of sar and agr were examined in three different Staphylococcus aureus strains by Northern blot analyses and by the use of reporter gene fusion experiments. Significant sigma(B) activity was detectable only in the clinical isolates MSSA1112 and Newman, carrying the wild-type rsbU allele, but not in the NCTC8325 derivative BB255, which is defective in rsbU. sigma(B) activity peaked in the late exponential phase and diminished towards the stationary phase when bacteria were grown in Luria-Bertani medium. Transcriptional analysis and a sarP1-sarP2-sarP3 (sarP1-P2-P3)-driven firefly luciferase (luc+) reporter gene fusion demonstrated a strong sigma(B) activity- and growth phase-dependent increase in sar expression that was totally absent in either rsbU or Delta rsbUVWsigB mutants. In contrast, expression of the agr locus, as measured by RNAIII levels and by an hldp::luc+ fusion, was found to be higher in the absence of sigma(B) activity, such as in rsbU or Delta rsbUVWsigB mutants, than in wild-type strains. Overexpression of sigma(B) in BB255 derivatives resulted in a clear increase in sarP1-P2-P3::luc+ expression as well as a strong decrease in hldp::luc+ expression. The data presented here suggest that sigma(B) increases sar expression while simultaneously reducing the RNAIII level in a growth phase-dependent manner.
Figures

References
-
- Balaban N, Goldkorn T, Nhan R T, Dang L B, Scott S, Ridgley R M, Rasooly A, Wright S C, Larrick J W, Rasooly R, Carlson J R. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–440. - PubMed
-
- Bischoff M, Roos M, Putnik J, Wada A, Glanzmann P, Giachino P, Vaudaux P, Berger-Bächi B. Involvement of multiple genetic loci in Staphylococcus aureus teicoplanin resistance. FEMS Microbiol Lett. 2001;194:77–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

