Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis
- PMID: 11489924
- PMCID: PMC209369
- DOI: 10.1172/JCI13662
Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis
Figures
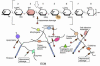
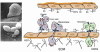
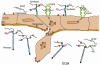
References
-
- Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. - PubMed
-
- Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
-
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. - PubMed
-
- Vlodavsky, I., Bar-Shavit, R., Korner, G., and Fuks, Z. 1993. Extracellular matrix-bound growth factors, enzymes and plasma proteins. In Basement membranes: cellular and molecular aspects. D.H. Rohrbach and R. Timpl, editors. Academic Press. Orlando, Florida, USA. 327–343.
-
- Vlodavsky I, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

