Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion
- PMID: 11489939
- PMCID: PMC209352
- DOI: 10.1172/JCI11294
Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion
Abstract
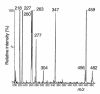
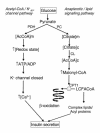
A female infant of nonconsanguineous Indian parents presented at 4 months with a hypoglycemic convulsion. Further episodes of hypoketotic hypoglycemia were associated with inappropriately elevated plasma insulin concentrations. However, unlike other children with hyperinsulinism, this patient had a persistently elevated blood spot hydroxybutyrylcarnitine concentration when fed, as well as when fasted. Measurement of the activity of L-3-hydroxyacyl-CoA dehydrogenase in cultured skin fibroblasts with acetoacetyl-CoA substrate showed reduced activity. In fibroblast mitochondria, the activity was less than 5% that of controls. Sequencing of the short-chain L-3-hydroxyacyl-CoA dehydrogenase (SCHAD) genomic DNA from the fibroblasts showed a homozygous mutation (C773T) changing proline to leucine at amino acid 258. Analysis of blood from the parents showed they were heterozygous for this mutation. Western blot studies showed undetectable levels of immunoreactive SCHAD protein in the child's fibroblasts. Expression studies showed that the P258L enzyme had no catalytic activity. We conclude that C773T is a disease-causing SCHAD mutation. This is the first defect in fatty acid beta-oxidation that has been associated with hyperinsulinism and raises interesting questions about the ways in which changes in fatty acid and ketone body metabolism modulate insulin secretion by the beta cell. The patient's hyperinsulinism was easily controlled with diazoxide and chlorothiazide.
Figures



References
-
- Vredendaal PJ, et al. Human short-chain L-3-hydroxyacyl-CoA dehydrogenase: cloning and characterization of the coding sequence. Biochem Biophys Res Commun. 1996;223:718–723. - PubMed
-
- Vredendaal PJ, et al. Structural organization of the human short-chain L-3-hydroxyacyl-CoA dehydrogenase gene. Mamm Genome. 1998;9:763–768. - PubMed
-
- Hammar H, Berne C. The activity of β-hydroxyacyl-CoA dehydrogenase in the pancreatic islets of hyperglycaemic mice. Diabetologia. 1970;.6:526–528. - PubMed
-
- Ågren A, Borg K, Brolin SE, Carlman J, Lundqvist G. Hydroxyacyl CoA dehydrogenase, an enzyme important in fat metabolism in different cell types in the Islets of Langerhans. Diabete Metab. 1977;3:169–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

